TUDelft/16 September 2008
From 2008.igem.org
| July | ||||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| 28 | 29 | 30 | 31 | |||
| August | ||||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | ||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 25 | 26 | 27 | 28 | 29 | 30 | 31 |
| September | ||||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| 29 | 30 | |||||
| October | ||||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | 4 | 5 | ||
| 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| 27 | 28 | 29 | 30 | 31 | ||
September 16th
Colony PCR 3A Assembly
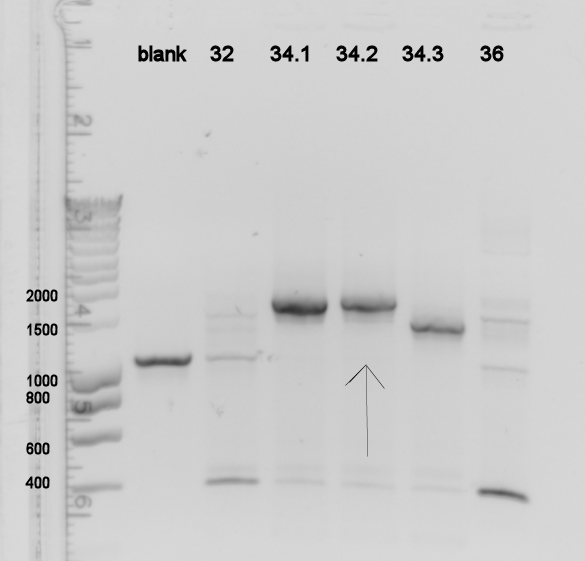
The transformation of the overnight ligation of the available parts K115029-K115036 didn't work on most plates. Only K115032 and K115034 had 1 and 3 colonies respectively. K115036 might have had a colony but was not very convincing. Colony PCR has been done on these spots, showing only K115034 was at the right size. New restrictions and ligations have been started for all parts K115029-K115036 (excluding K115030 and K115035 as not all DNA has arrived yet), again we will use vector pSB1AT3. The blank showed a curious result, but later results showed our water is clean. We did stir a toothpick through the colony PCR mix blank sample, which might have been infected somehow.
Gel of the E. coli genes
It seems most of the transformants were closed vectors without insert. Currently, we could test more colonies and hope there are some good ones in there. On the other hand, it may be worthwhile and a more certain way to go to order new primers (containing the whole prefix and suffix, allowing for PstI & EcoRI cutting) to avoid the assembly problem we are now facing. XbaI and SpeI digestions yield compatible sticky ends...
 "
"