Team:Chiba/Project/Experiments:Sender Crosstalk
From 2008.igem.org
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
Sender Crosstalk
Design
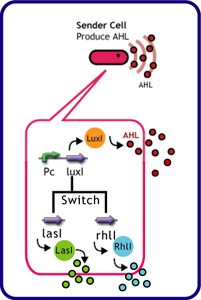
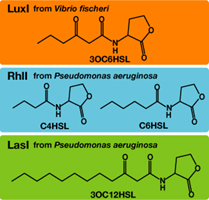
Each species has their own LuxI-type proteins,which synthesize their specific autoinducers, AHLs. AHLs produced by different LuxI-type proteins differ only in the length of the acyl-chain moiety and substitution at position C-3(4).LuxR,which is original for Vibrio fischeri, is activated by its cognate autoinducer, 3OC6HSL. However, LuxR is also activated by non-endogenous molecules, C4HSL, C6HSL, and 3OC12HSL. Activation by non-endogenous molecules requires a higher signal concentration (1),(2). This results in slower activation of receivers, when AHL concentration is increasing.
Experiments
The purpose of this experiment was to create delays in communication time using cross-talk between non-specific signals. We used the following genes for this experiment.
| senders (cell: XL10-Gold or JW1908) | receiver (cell: JW1908) |
| *plac+rbs+LasI (pSB1AK3) | *BBa_T9002 (Express GFP in response to AHL) (pSB1A3) |
| *plac+rbs+RhlI (pSB1AK3) | |
| *BBa_K084009 | |
| *plac+rbs+LuxI (pSB1AK3) | |
| *BBa_K084014 | |
| *BBa_S03623(ptet+rbs+LuxI(LVA)) |
Details
Method
To characterize quorum sensing crosstalk, constitutive AHL senders were mixed with constitutive receivers and measure fluorescence intensity.
- Transformed Senders into E. coli strains (XL10Gold or JW1908) and Receiver into E. coli strain (JW1908).
- Inoculated them independently in liquid media. Incubated at 37°C for 12hours.
- Mixed them.
- Incubated at 37°C or 30°C.
- Measured intensity of green fluorescence (485 nm (excitation) and 527 nm (emission)) at regular time intervals.
Results & Discussion
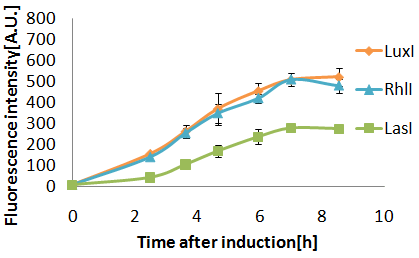
Senders(XL10Gold), T9002(JW1908)@30°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
When incubated at 30°C,E.coli strain(XL10Gold) transformed with the LasI genes produced more AHL than at 37°C.
(Green fluorescence intensity after incubating for 8 hours was:
- 163 at 37°C.
- 245 at 30°C.
3OC12HSL produced by LasI protein(expressed byBBa_K084007 and 3OC6HSL produced by LuxI protein(expressed byBBa_K0840012) both activated LuxR protein and cousese gfp expression.The increase of green fluorescence intensity caused by 3OC12HSL was more gradualy than that caused by 3OC6HSL,time before fluorescence intensity reached at 200 was 2.5 hours longer than that of the 3OC6HSL.
Future plans
- Reduce quantity of expression of an enzyme composing AHL
- Replace medium RBS with weak one.
- Alter the copy number of plasmids in the sender into low.
- To make the slope of transfer curve of our device to be steep:
- Introduce Positive Feedback Loop into our genetic circuit.
Other Experiments
Visual Judgement
fluorescence intensity was from 150 to 200 when the 2 mL of reaction culture in a test tube.
Senders(JW1908), T9002(JW1908)@37°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
Senders(JW1908),T9002(JW1908)@30°C,Sender(μL):Receiver(μL)=500:500,100:1000,10:1000
Senders(XL10Gold),T9002(JW1908)@37°C, Sender(μL):Receiver(μL)=500:500,100:1000,10:1000
Senders(XL10Gold), T9002(JW1908)@25°C, Sender(μL):Receiver(μL)=500:500, 100:1000, 10:1000
Demo ~Senders~
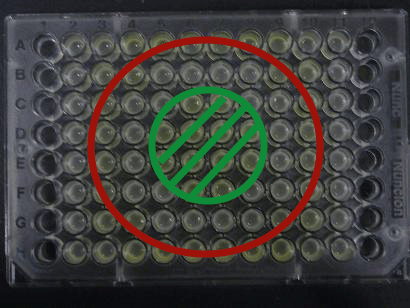
We experimentally tested the sender genes LuxI and LasI which produce the greatest time difference. We mixed bacteria (XL10Gold) transformed with either the LuxI or LasI genes and bacteria (of the strain JW1908) transformed with the LuxR gene at a 1:1 ratio and visually observed GFP fluorescence.
Results
Green region: sender=LuxI (50 uL), Red circular region:sender culture containing LasI gene(50 μL).Receiver culture containing LuxR-plux-gfp gene (50 μL)
-->more about Demo experiments detail
references
- M.K Winson et al.:Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing.FEMS Microbiology Letters 163 (1998) 185-192
- BBa_F2620:Specificity
- Paul Williams.:Quorum sensing, communication and cross-kingdom signalling in the bacterial world.Microbiology 153 (2007), 3923-3938
- Michiko E. Taga. Bonnie L.Bassler.:Chemical communication among bacteria.PNAS.November 25, 2003,100.suppl.2
 "
"