Team:Edinburgh/Plan/StarchSynthesis
From 2008.igem.org
Contents |
Starch synthesis
Glucose must be taken up by the cells and synthesised first into glycogen and then starch.
Genes
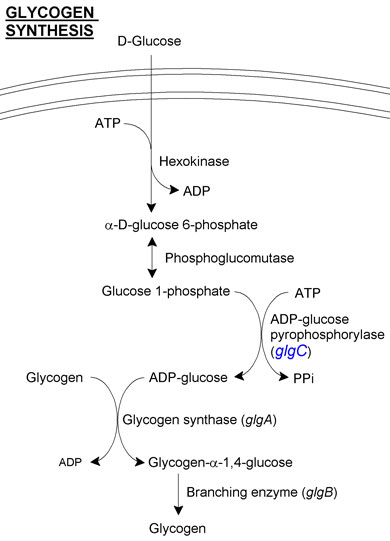
Glycogen synthesis
E. coli and B. subtilis are natively able to synthesise glycogen from starch (see Figure 1 for E. coli's native pathway). However, we want to upregulate this pathway:
- The gene glgC (ADP-glucose pyrophosphorylase, catalysing the convertion glucose 1-phosphate and ATP to ADP-glucose and PPi) is responsible for the most rate-limiting step of glycogen synthesis in E. coli. This is because the protein is negatively regulated by 5'-AMP, ADP or orthophosphate. The glgC16 mutant has the substitution G336D to glgC, and has been reported to increase the yield of glycogen. This is due in part to the loss of allosteric inhibition and is also due to increased activity in the absence of its activator molecule, fructose 1,6 bis-phosphate.
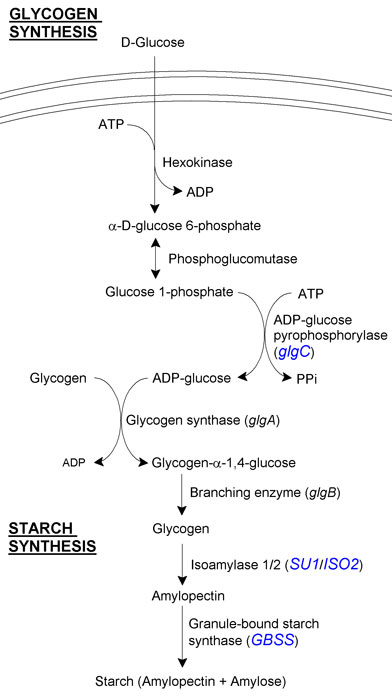
Starch synthesis
Starch is composed of amylopectin, a polyglucan linked by α-1,4 and α-1,6 bonds, and amylose, a derivative of amylopectin with fewer α-1,6 linkages and thus a more linear structure. Synthesising starch from glycogen requires just a few steps and genes:
- SU1 (isoamylase 1) from Zea mays. This associates with isoamylase 2 and together they are responsible for the conversion of glycogen to amylopectin. We decided to use an isoamylase from a plant that naturally accumulates a lot of starch, and it was for this reason that we settled on Z. mays.
- ISO2 (isoamylase 2) from Zea mays. This associates with SU1 to convert glycogen to amylopectin.
- GBS1 (granule-bound starch synthase) from Arabidopsis thaliana. This converts some amylopectin into amylose, and thus is responsible for the final step in starch production. It also compacts the starch into a intracellular granule.
Starch synthase operon
We planned for glgC, SU1, ISO2 and GBS1 BioBricksTM to be placed as a single operon in that order. They should be under the control of an endogenously expressed promoter. However, to test proof of concept we decided instead have them under the control of Plac (BBa_J33207) to allow some control over expression.
Overview
- In blue are the genes which we intended to manipulate.
 "
"