Team:Heidelberg/Notebook/Killing I/Notebook/week8
From 2008.igem.org


| << Week 7 | Overview | Week 9 >> |
|---|
Week 8
Contents |
Monday, 09/22/08
oriT
- there are already OriTs available at the registry, OriTs for F and for R - helper plasmids. They are mostly combined with transposons for mutagenesis experiments. but there are few parts with a confmirmed sequence:
- J01003: OriT sequence in pSB1A2 (278 bp)
- I714031: OriT sequence (of R 751 plasmid) in pSB1A2 (371 bp)
- both of the parts did not pass the iGEM quality control - no insert could be seen after restriction digestion. we will transform both OriTs and make conjugation tests to see if they are functional. If not, we would still be the first group to add a functional OriT for R-helper plasmids.
- first attempt to transform these OriTs out of the registry did not work...
Phage cloning strategy one
- miniprep of possible lambda DNA (from colonies of ligation)
- nanodrop evaluation of minipreps
- 2: 186,0 ng/µl
- 3: 269,5 ng/µl
- 7: 244,5 ng/µl
- 9: 264,5 ng/µl
- 10: 245,5 ng/µl
- all assays were clean ~1,87
- digestion with SpeI/XmaI (last lane: SpeI/ApaLI)
- --> seems not to be a lambda phage but rather religated pBluescript
- Mutagnesis PCRs of pBlue::insert to delete the XbaI restriction sites
- digestion with DpnI, 2h at 37°C
- transformation in TOP10
- inoculation of I20260::CmR 1-3, reference promotor, pBlue::Insert, pBluescript instead of Top10 in 5ml LB
Tuesday, 09/23/08
Phage cloning strategy one
- preparation of in vitro packaging media
- supplemented LB-Broth
- supplemented Agar
- phage dilution buffer
- for detailed protocols see main page - protocols
- GFP-measurement with the TECAN of TOP10 including pBluescript with the insert
- the GFP::CmR construct in pBluescript does not express GFP!!!
- bicistronic gene assembly does not work!!
- inoculation of the cells possible containing the lambda phage for GFP meausurement
Wednesday, 09/24/08
Phage cloning strategy one
- digestion of the lambda phage with XbaI, XhoI (protocol described previously)
- GFP measurement with TECAN of the cells containing possibly the lambda phage
- no GFP expression could be observed!
Thursday, 09/25/08
Cloning oriT in standard plasmid
- PCR of oriT wirh oriT_pre and oriT_suf
- templates: original oriT, pBluescript oriT
1µl DNA 2µl oriT_pre 2µl oriT_suf 25µl Phusion Master Mix 20µl water
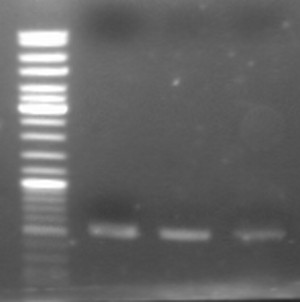
- PCR product on gel:
- expected size: ~500bp
- --> looks good
- PCR purification
- digestion with PstI and XbaI
30µl DNA 1,5µl PstI 1,5µl XbaI 4µl BSA 4µl NEB3
- PCR purification kit
- digestion of T9002 with PstI, XbaI, NcoI to get pSB1A3
3µl DNA 4µl NEB3 4µl BSA 2µl PstI 2µl XbaI 2µl NcoI 23µl water ----- 40µl
- expected sizes: 711, 1260, 2131 (pSB1A3)
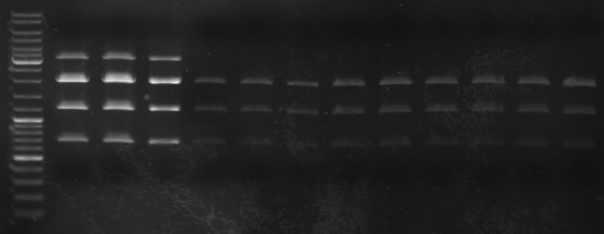
- gel
- lane 0: ladder
- lane 1-3: maxiprep
- lane 4-6: mini1
- lane 7-9: mini2
- lane 10-12: mini3
- cutted out 2131bp band and gel purification kit
Phage cloning strategy one
new cloning attempt
- digestion of the insert in pBluescript with XhoI, KpnI, ApaLI --> 3363 rest insert
DNA 1,5 µl XhoI 1,5µl KnpI 1µl ApaLI 1µl NEB1 3µl BSA 3µl water 19µl
- digestion of the insert in pBluescript with XbaI, KpnI --> 479 oriT
DNA 2µl XbaI 1µl KpnI 2µl NEB2 3µl BSA 3µl water 19µl
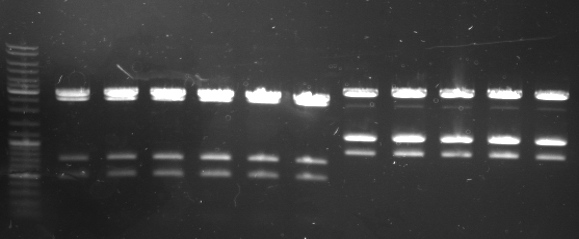
- Gel
- lane0: ladder
- lane1-6: oriT: 479 (10,59,479,722,2583,2887)
- lane7-11: rest insert: 3363 (11,905,1215,1246,3363)
- --> looks good
- extraction of the lambda backbone with XbaI and XhoI
- gel
- gel purification kit
- ligation of lambda phage big fragment + oriT digested with XbaI, KpnI + big fragment (GAM, GFP, CmR) digested with XhoI, KpnI, ApalI) over night
Transformation
- transformation of 8 old lambda phage ligations a-g from chris
- used cells: from the "ig-box"
- incubate at 28° to see wether colonies appear
Extraction of phages
- phage extraction with 5 ml phage dilution buffer on LB plate with plaques 5h at RT
- titer evaluation of phages (Anna)
Friday, 09/26/08
- gel extraction of the standard plasmid backbones
- transformation of 8 different (new) ligations on two Cm-plates each (incubate at 28°)
transformation of lambda overnight ligations a-g
- each ligation product was transformed with the new competent cells from thurday and with the old cells in the "ig-box"
- plated out on Cm plates
in vitro packaging kit
- with overnighht ligation a and g
- plated out dilutions: 10^-1, 10^-2, 10^-4, 10^-5, 10^-6 on LB plates with LB top agar
- plated out dilutions: 10^-1, 10^-2, 10^-4 on Cm plates with Cm top agar
Mutagenese PCR with mut_kpn1_pBlue
5 µl Pfu Buffer 5x 1 µl mut_kpn1_pBlue_fw 1 µl mut_kpn1_pBlue_rev 1,5 µl dNTPs 2 µl template DNA (pBlue with insert dilution: 1:100) 1 µl Pfu turbo 38,5 µl water ------ 50 µl
PCR protocol 95°C 30s 95°C 30s | 55°C 30s | 15x 68°C 14min | 4°C for ever
Sequencing of miniprep 6 and 7.2 with lambda_insert_fw
- sequencing was not possible
| << Week 7 | Overview | Week 9 >> |
|---|
 "
"