Team:University of Lethbridge/Notebook/Project1August
From 2008.igem.org
Back to The University of Lethbridge Main Notebook
Contents |
August 13, 2008
Selina, Munima
Objective: Second attempt at making chemically competent RP1616 cells and transforming them with pTopp and pSB1A7. These protocols are modified Sambrook protocols from the Mosimann Lab.
Preparation of Chemically Competent Cells in CaCl2
Protocol (adapted from Sambrook & Russell, (2001) Molecular Cloning: A Laboratory Manual 3rd Edition. Protocol 25 Vol. 1; 1.117):
1. Inoculated 100 mL liquid LB media using 100 uL of glycerol stocked RP1616 cells from iGEM07. Incubated at 37C
for 4 hours. Measured A600 to be 0.666 (ideal A600 = 0.4 - 0.6).
2. Transferred cells to sterile, ice-cold 35 mL centrifuge tube (NOT FALCON TUBE). Cooled culture on ice for
10 minutes.
3. Pelleted cells by centrifugation at 2000 rpm for 5 min and decanted supernatant.
4. Resuspended gently, using Pasteur pipette, in 20 mL of ice-cold 80 mM MgCl2-20 mM CaCl2 solution.
5. Centrifuged cells at 2500 rpm for 5 min and decanted supernatant.
6. Resuspended cells, by gentle swirling, in 2 mL of 100 mM CaCl2.
Created one 30 % glycerol stock and two (accidentally) 50% glycerol stocks. Placed temporarily in Steve's -80C freezer in Selina's box.
___
Transformation of CaCl2 Competent Cells with Plasmid DNA
Attempted to transform pTopp and pSB1A7 (positive control) into (hopefully) CaCl2 competent RP1616 cells.
Protocol:
1. Added plasmid solutions pTopp (5, 10 or 20 uL) or pSB1A7 (5, 10 uL)into 200 uL of chem. competent cells.
-pTopp plasmid prep from ______, pSB1A7 from ______
2. Incubated cells on ice for 30 minutes.
3. Heat shocked cells for 2 minutes at 42 C (for 1.5 mL microcentrifuge tube).
4. Incubated on ice for 5 minutes.
5. Added 1.0 mL of pre-warmed LB media.
6. Incubated at 37 C for 60 minutes with gentle shaking (~100 rpm).
7. Pelleted cells at 6000g for 3 minutes (7000 rpm) and removed 700 uL. Resuspended the cell pellet in the remaining
300 uL by gentle pipetting.
8. Spread the 300 uL suspension on an LB + Amp plate.
9. Incubated overnight at 37 C.
August 14, 2008
Selina
Checked transformation plates (Aug. 12, RP1616 + pTopp or pSB1A7 plasmid) after 14.5 hours.
No growth on any of the plates.
August 15, 2008
Munima
Objective: Prepare cells for electroporation/chemical competency.
Streaked RP1616 (from glycerol stock) onto LB plate and inoculated 5 mL LB liquid media tube with the same culture. Streaked glycerol stocked pSB1A7 onto LB + Amp plate - will plasmid prep to replenish pSB1A7 stock in the -20 C freezer.
August 16, 2008
Selina
Objective: Reattempt to create CaCl2 chemically competent RP1616 E. coli cells, following Sambrook's protocol directly, instead of using a modified version from the Mosimann lab
Modifications to Aug. 13 protocol:
-Used cell culture of OD600 = 0.503 -Final resuspension of 1 mL CaCl2 per ~35 mL cells
Streaked CaCl2 treated cells on LB plate directly after competency treatment and placed at 37C overnight
Created glycerol stocks labeled 'CaCl2 treated RP1616' and placed in HJ's -80C
Selina
Objective: Test CaCl2 treated cells (Aug. 16) for competency
Used Sambrook's direct-from-CaCl2-treatment transformation protocol.
Volumes used for transformations:
-100 mL CaCl2 cell suspension per transformation
-Varying amounts of plasmid solution (Sambrook advises <5% of cell culture volume and max 25ng/50mL cell culture
-pTopp: 1 uL, 2.5 uL, 5 uL
-pSB1A7: 1 uL, 2.5 uL, 5 uL
-pUC19 (positive control): 1 uL, 2.5 uL
-Plated resulting cells on LB + Amp plates and left overnight at 37C
Munima
Objective: Reattempt electroporation of RP1616 with pSB1A7 and pTopp, using the same procedure (with minor modifications) as used on July 16, 2008.
Machine: Eppendorf Electroporator 2510
Protocol:
1. Make a 1/5 dilution of cells from last night's culture tube into a new 5 mL LB liquid media tube. Leave dilution in shaker incubator at 37 C (300RPM) for approximately 4 hours. 2. Transfer 1.5 mL of the 1/5 dilution into two microcentrifuge tubes (one for each plasmid used). 3. Spin down at max for 1 minute. 4. Pour off supernatant. Resuspended in 1 mL of ice cold water to wash (2x). 5. Resuspend in 100 uL of 10% glycerol in water. 6. Add 2 uL of plasmid. Sit for 10 min on ice. 7. Poured mixture into prechilled 0.1 cm cuvette. 8. Electroporate with 1.5 kV for ~2.4 msec. 9. Immediately add 1 mL of SOC media. 10. Transfer to 15 mL Falcon tubes. 11. Shaker incubate at 300 RPM and 37 C for approximately 1 hour. 12. Plate onto LB + antibiotic plates (Ours were LB + Amp; [Amp]=100 ug/mL) 13. Incubate plates at 37 C for no more than 16 hours.
Plated 50 uL and 100 uL of each RP1616+plasmid culture onto LB + amp plates.
Did two batches of this electroporation protocol, so had eight plates in total. For the first batch, forgot to let the cells sit for 10 min on ice before applying electric field after adding pSB1A7.
August 17, 2008
Munima
Checked plates for the results of my electroporation and Selina's chemical competency at 8am (16 hours later).
Plates from electroporation had strange tiny, mist-like dots. The LB plate with CaCl2 treated RP1616 cells (before any transformation) had normal looking growth with correct morphology. Basically electroporation and chemical comp. transformation were unsuccessful. Placed plates (parafilmed) in 4C fridge.
Future direction: try electroporation and/or chemical competency with wild type E. coli. as positive control for competency experiments
August 18, 2008
Selina
Inoculated 4 x 5 mL LB + Amp culture tubes with E. coli (pSB1A7) colonies from Aug. 15/08 (in fridge)
Marked 'used' colonies on plate with circle and black 'x' covering colony location.
August 19, 2008
Munima, Christa, Sebastian
Plasmid prepped pSB1A7 from culture tubes inoculated by Selina yesterday. Used Qiaprep Spin Miniprep Kit. Stored four microcentrifuge tubes of 50 uL each in the iGEM -20 C freezer. They are labelled as "pSB1A7 DH5a Aug. 19, 2008".
August 21, 2008
Munima, Christa
Objective: Begin working on part. Insert CheZ into pSB1A7.
Set up a PCR for CheZ.
Reaction conditions:
- Initial denaturation: 98 C (4 min) - Denaturation: 98 C (30 sec) - Annealing: 47 C (30 sec) - Extension: 72 C (30 sec) - Final Extension: 72 C (7 min); 4 C (hold)
35 cycles for denaturation, annealing and extension steps.
Set up 1.5 reactions (1 at 50 uL and the other was less) for CheZ and a negative control (50 uL).
August 22, 2008
Christa, Munima, Nathan Puhl, Roxanne
Objective: Run a gel to see if PCR worked and do a gel extraction of the gene to prepare it for insertion into pSB1A7.
(Similar info in the General Lab: August notebook) Ran a 1% agarose gel (15 uL x 3 wells) and confirmed that the CheZ band was a the correct size for the gene. No bands were present for the negative control. Could not take a picture because the camera was not working. Performed a gel extraction using Qiagen MiniElute Gel Extraction kit. final volume after gel extraction was 10 uL. Ran another 1% agarose gel (1 uL of sample) to confirm that the gel extraction worked. Estimate that the concentration of CheZ is ~80 ng/uL.
August 24, 2008
Selina
Objective: Retry CaCl2 induced competency experiment, running a proper positive control of non-competent E. coli DH5a strain
Procedure:
-Inoculated a 5 mL LB culture tube with E. coli DH5a (8 pm) and left to incubate overnight in Steve's 37 C
-E. coli should lose competency with cell growth in LB
-Also inoculated a 5 mL LB culture tube with RP1616 at the same time, also left overnight at 37 C
August 25, 2008
Selina
Objective: CaCl2 competency experiment cont.
Procedure:
1. Inoculated 200 mL LB culture with 1 mL of overnight E. coli culture (Aug. 24) (for both DH5a and RP1616 strains) 2. Left to incubate for ~1.5 hours, at which time the OD600 = 0.347 (ideal = 0.4) 3. Transferred 30 mL to sterile centrifuge tube, left on ice for long time - some interlab travelling time.... probably around 40 mins? (ideal = 10 mins) 4. Spun in Ute's centrifuge at 3000 rpm for 5 mins 5. Decanted media and dripped out excess onto paper towel (suggested by Sambrook) and added 15 mL ice-cold sterile 80 mM MgCl2-20 mM CaCl2 6. Spun at 3500 rpm for 5 mins 7. Decanted and added 1 mL 100 mM CaCl2.
note: almost all out of MgCl2 and CaCl2 autoclaved solutions! need to make more for next go-around!
Jaden, Selina
Objective: Attempted transformation of CaCl2 treated cells (positive control and RP1616)
Set-up:
-Competency controls: -DH5a-CaCl2-competent + pUC19 = positive -Transformation controls: -DH5a-CaCl2-competent + nothing = negative on LB + Amp, positive on LB (not yet run) -RP1616-CaCl2-competent + nothing = negative on LB + Amp, positive on LB (not yet run) -DH5a-CaCl2-competent + pUC19 = positive on LB + Amp -DH5a-CaCl2-competent + pTopp = positive on LB + Amp -RP1616-CaCl2-competent + pUC19 = positive on LB + Amp -Fresh DH5a + pUC19 = positive control -Experimental: -RP1616-CaCl2-competent + pTopp
Amounts used:
- CaCl2 suspended cells = 200 uL - Fresh DH5a cell culture = 25 uL - Plasmid = 1 uL for CaCl2 treated cells, 0.7 uL for fresh DH5a
Followed protocol used by Ute's lab. Plated a final volume of 250 uL for each reaction.
August 26, 2008
Selina
Checked transformation plates.
Results:
-DH5a-CaCl2-competent + nothing on LB + Amp = no growth :) -RP1616-CaCl2-competent + nothing on LB + Amp = no growth :) -DH5a-CaCl2-competent + pUC19 on LB + Amp = some colonies :) -DH5a-CaCl2-competent + pTopp on LB + Amp = even more colonies :) -RP1616-CaCl2-competent + pUC19 on LB + Amp = no colonies :( -Fresh DH5a + pUC19 = lots of colonies :) -RP1616-CaCl2-competent + pTopp = nothing :(
Interpretation:
-All controls worked, but experiments failed
-Possible explanations?
-DH5a was not actually made uncompetent - could have run another negative control without CaCl2
treatment... unlikely reason though
-RP1616 are heat sensitive? try plating on regular LB as another positive control
-Statistically more unfavorable - increase reaction volume? (unlikely b/c of earlier attempts, but will try)
-????
Plated 10 uL heat-shocked RP1616 (ie: transformation w/o DNA) on LB. Left at 37 C overnight.
Plated rest of RP1616 transformations on LB + amp. Volume was initially ~800 uL. Spun down, removed 600 uL and plated the rest. Left at 37 C overnight.
Parafilmed E. coli DH5a CaCl2 - pTopp and pUC19 control transformation plates and placed in 4C fridge.
August 27, 2008
Selina
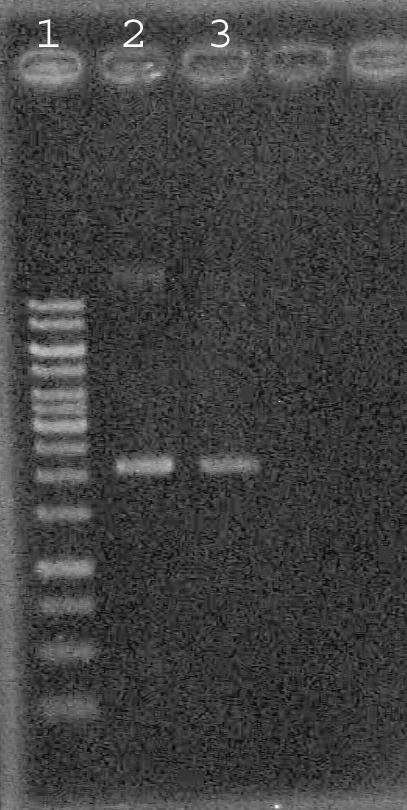
Ran last year's GFP complete plasmid prep on 1% TAE agarose gel
Lane 1: GeneRuler 1 kb ladder
Lane 2: GFP complete prep 1
Lane 3: " " " 2
Christa, Munima
Objective: Attempt electroporation of RP1616 with "GFP complete" plasmid.
Inoculated RP1616 into LB liquid media. Left on shaker incubator to grow overnight at 37 C (300 rpm).
Viewed image of "GFP complete" plasmid gel run by Selina. Determined the plasmid concentration to be ~15 ng/uL.
August 28, 2008
Christa, Munima
Objective: Reattempt electroporation of RP1616 with "GFP complete" plasmid, using the same procedure (with minor modifications) as used on July 16 and August 17, 2008. Since results have always looked inconclusive, this plasmid will be used to see if any colonies immediately fluoresce green, as an implication of a successful transformation.
Machine: Eppendorf Electroporator 2510
Protocol:
1. Make a 1/5 dilution of cells from last night's culture tube into a new 5 mL LB liquid media tube. Leave dilution in shaker
incubator at 37 C (300RPM) for approximately 4 hours. (Munima - 9am)
2. Transfer 1.5 mL of the 1/5 dilution into two microcentrifuge tubes (one for each tube of GFP complete plasmid).
(Steps 2-11 by Christa and Munima - starting at 10:30am).
3. Spin down at max for 1 minute.
4. Pour off supernatant. Resuspended in 1 mL of ice cold water to wash (2x).
5. Resuspend in 100 uL of 10% glycerol in water.
6. Add 2 uL of plasmid. Sit for 10 min on ice.
7. Poured mixture into prechilled 0.1 cm cuvette.
8. Electroporate with 1.5 kV for ~2.4 msec.
9. Immediately add 1 mL of SOC media.
10. Transfer to 15 mL Falcon tubes.
11. Shaker incubate at 300 RPM and 37 C for approximately 1 hour.
12. Plate onto LB + antibiotic plates (Ours were LB + Amp; [Amp]=100 ug/mL) (Christa - 12:30pm)
13. Incubate plates at 37 C.
Plated 50 uL and 100 uL of each RP1616+plasmid culture onto LB + Amp plates.
August 29, 2008
Munima
Checked results of electroporation. Plates had similar small colonies as the results of other electroporation attempts. No colonies immediately seem to fluroesce green. Futher examination required. Plates were parafilmed and stored in the iGEM 4 C fridge.
 "
"