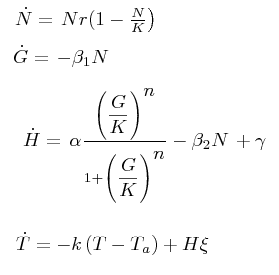Team:Valencia/Project/Modeling
From 2008.igem.org
| Line 40: | Line 40: | ||
==Black box model of the temperature increase by the thermogenin== | ==Black box model of the temperature increase by the thermogenin== | ||
| - | [[Image:Ecuacion_efectivo.jpg]] | + | [[Image:Ecuacion_efectivo.jpg|230px]] |
==References== | ==References== | ||
Revision as of 10:28, 8 September 2008
Contents |
Oxidative phosphorylation model coupled with thermogenine expression
Our project consist of implementing a controlled heating system inside Saccharomyces cerevisiae. In order to obtain this we are going to use a mitocondrial membrane protein known as thermogenin used by mamalians cells to increase its temperature.
The working principle of thermogenin is based on the respiratory electron transport chain. The way in which the thermogenin increases the temperature of the system can be described approximately as follows: It produces a hole on the mitocondrial membrane which makes difficult the production of a proton gradient in the membrane. The release of the mitocondrial proton gradient increases the temperature. (ficar referencia)
In order to describe the system three complementary models have been developed :
- Black box model of the temperature increase by the thermogenin.
- Detailed model of the respiratory electron transport chain.
- Regulatory model of the thermogenin expresion.
Theoretical evaluation of the idea
To evaluate the reliability of the idea we are going to estimate the maximal attainable temperature (obviously, the minimal temperature value is the corresponding to the culture conditions). This means to estimate the maximum heat production. The maximal temperature can be determined by supposing that the proton motrive force is used solely in heat production mediated by thermogenine. The following calculations were made taking into account this assumption.
To calculate the energy produced per proton dissipated through the thermogenine we used the published rate ( Milakovik et al 2005) of ATP flow as an estimation of the Saccharomyces cerevisiae real value.
17.5 nmol/min = 2.92 • 10 -10 mol/s = 1.76 •1014 ATP particles/s
If ATP synthase needs four protons to produce one molecule of ATP, then we can easily calculate the proton flow through complex V.
7.03 •1014 p+/s
The free energy associated to a single proton being dissipated through the ATP synthase is 20 Kj/mol. Balancing the units we get the energy dissipated per proton:
3.32 •1023 Kj/p+
The energy flow is calculated by multiplying both figures and adjusting the result to the proper units:
2.33 •10-5 J/s
Black box model of the temperature increase by the thermogenin
References
Milakovic T. and Johnson G. V. W.2005. Mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin. The Journal of Biological Chemistry. 280:30773-30782.
 "
"