Team:BCCS-Bristol/Calendar-Notebook/6 August 2008
From 2008.igem.org
(New page: ==BioBrick Transformation== Last Thursday, the primers [http://partsregistry.org/wiki/index.php/Part:BBa_G00100 VF2] and [http://partsregistry.org/Part:BBa_G00101 VR] arrived. These pri...) |
|||
| Line 3: | Line 3: | ||
Last Thursday, the primers [http://partsregistry.org/wiki/index.php/Part:BBa_G00100 VF2] and [http://partsregistry.org/Part:BBa_G00101 VR] arrived. These primers allow a confirmation of a succesful transformation of most BioBrick parts by means of PCR. The resulting fragment length depends on the specific BioBrick DNA. | Last Thursday, the primers [http://partsregistry.org/wiki/index.php/Part:BBa_G00100 VF2] and [http://partsregistry.org/Part:BBa_G00101 VR] arrived. These primers allow a confirmation of a succesful transformation of most BioBrick parts by means of PCR. The resulting fragment length depends on the specific BioBrick DNA. | ||
| - | Using VF2 and VR, a colony PCR are was conducted with all colonies that were obtained with chemical competent cells so far. Two colonies with the [http://partsregistry.org/Part:BBa_J63005 yeast ADH1 promoter] (No. 1 and 2, see left photo) and three colonies with a [http://partsregistry.org/Part:BBa_E0240 GFP generator] (No. 3-5, see left photo) were identified. | + | Using VF2 and VR, a colony PCR are was conducted with all colonies that were obtained with chemical competent cells so far. Two colonies with the [http://partsregistry.org/Part:BBa_J63005 yeast ADH1 promoter] (No. 1 and 2, see left photo) and three colonies with a [http://partsregistry.org/Part:BBa_E0240 GFP generator] (No. 3-5, see left photo) were identified as positive. Thus, all colonies are positive, but the transformation efficiency is too low, since the result was only one or zero colonies per transformation attempt. |
| + | |||
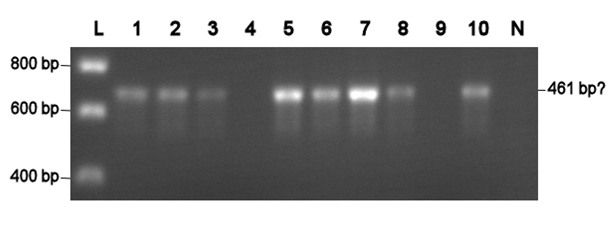
| + | Electroporation should give higher efficiencies. An attempt with a new BioBrick ([http://partsregistry.org/Part:BBa_J63002 ADH1 terminator]) resulted in 69 colonies! Ten of them were analysed using PCR. The resulting fragment should have a length of 461 bp, but from the eigth positive colonies fragments between 650-750 bp were obtained (see right photo). This is probably due to a mutation which might result in a different binding location for one of the primers. Another explanation might be that the denoted length is wrong. Since this BioBrick is not important for our project, it will be disregarded. | ||
| + | |||
| + | {| align="center" | ||
| + | | | ||
| + | [[Image:BCCS-080801-colony pcr BBa J63005+BBa E0240 labeled.png | 300px]] | ||
| + | [[Image:BCCS-080804 colony pcr BBa J63002 labeled.png | 400px]] | ||
| + | |} | ||
| + | |||
| + | {| align="center" | ||
| + | | | ||
| + | L= HyperLadder I (BIOLINE) | ||
| + | |||
| + | N= Negative control | ||
| + | |} | ||
| + | |||
| + | The transformation efficiency of the electroportation was significant higher than of the chemical competent cells. Therefore, an own stock of electric competent cells was made. | ||
Revision as of 18:11, 6 August 2008
BioBrick Transformation
Last Thursday, the primers [http://partsregistry.org/wiki/index.php/Part:BBa_G00100 VF2] and [http://partsregistry.org/Part:BBa_G00101 VR] arrived. These primers allow a confirmation of a succesful transformation of most BioBrick parts by means of PCR. The resulting fragment length depends on the specific BioBrick DNA.
Using VF2 and VR, a colony PCR are was conducted with all colonies that were obtained with chemical competent cells so far. Two colonies with the [http://partsregistry.org/Part:BBa_J63005 yeast ADH1 promoter] (No. 1 and 2, see left photo) and three colonies with a [http://partsregistry.org/Part:BBa_E0240 GFP generator] (No. 3-5, see left photo) were identified as positive. Thus, all colonies are positive, but the transformation efficiency is too low, since the result was only one or zero colonies per transformation attempt.
Electroporation should give higher efficiencies. An attempt with a new BioBrick ([http://partsregistry.org/Part:BBa_J63002 ADH1 terminator]) resulted in 69 colonies! Ten of them were analysed using PCR. The resulting fragment should have a length of 461 bp, but from the eigth positive colonies fragments between 650-750 bp were obtained (see right photo). This is probably due to a mutation which might result in a different binding location for one of the primers. Another explanation might be that the denoted length is wrong. Since this BioBrick is not important for our project, it will be disregarded.
|
L= HyperLadder I (BIOLINE) N= Negative control |
The transformation efficiency of the electroportation was significant higher than of the chemical competent cells. Therefore, an own stock of electric competent cells was made.
 "
"