Team:TUDelft/Temperature results
From 2008.igem.org
Contents |
Results
Assembly of BBa_K115012 and BBa_K115029 - BBa_K115036
The first challenge of this project was to assemble all the devices we wanted to measure. An overview of these devices can be found here. For the actual construction of all devices the 3A assembly strategy was chosen. A complete protocol of this strategy can be found on [http://openwetware.org/wiki/Synthetic_Biology:BioBricks/3A_assembly OpenWetWare]. A schematic representation of this assembly method is depicted in figure 1A and 1B.
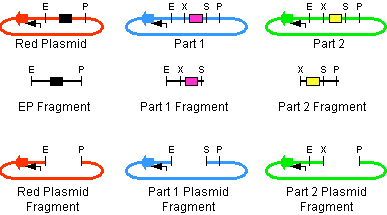
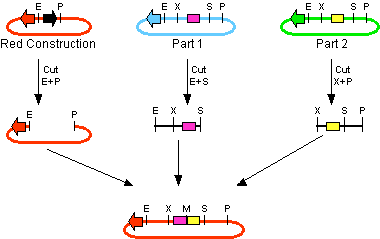
For the succesful use of this assembly, it is essential that the DB3.1 Escherichia coli strain is not used as it tolerates the presence of the CcdB gene. Throughout the project we used commercial Top10 cells of Invitrogen that were made competent chemically. Furthermore, it is important to note that the XbaI and SpeI restriction enzymes generate compatible sticky ends. Assuming that we want to place two BioBrick parts behind each other in one plasmid, but these are present in seperate plasmids before assembly (as is the case in Figure 1A, parts are depicted in purple and yellow in the blue and green plasmid, respectively). 3A assembly can be conducted by taking an empty plasmid that has a different antibiotic resistence as the other two plasmids (red in figure 1A and B, CcdB is present). Three seperate restriction reactions should be started as shown in figure 1A. After cleaning up the restriction reactions they are mixed and ligated together. Parts can be ligated in several ways:
- The CcdB gene can be ligated back in the vector - If this happens, cells will not grow after transfection, as the E. coli strain used should not be CcdB gene tolerant.
- Other parts can be ligated back in the vector - Other plasmids should not have the same antibiotic resistence as the red plasmid, in other words, these are selected for.
- Ligation as depicted in figure 1B - This is the ligation we are looking for and it should yield colonies after transformation.
- Other combinations of backbones and/or parts - It is possible other combinations (e.g. the three backbones together) form a plasmid that yields colonies, these parts will be much larger as the ones formed in option 3. Colony PCR's are performed afterwards to screen for these.
In the end, the result on the gel of the colony PCR product is conclusive in terms of the succes of the 3A assembly. For all our parts at least three of the 3A assemblies (first promoter + ribosome binding site and luciferase + terminators, afterwards the result of these two together) gave our final product. An overview of the result on the gels can be found here. We succeeded in getting colonies that gave the correct band size on the gel for [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2008&group=TUDelft BBa_K115012 and BBa_K115029 - BBa_K115036]. The parts constructed and the temperature at which they are supposed to switch are depicted in table 1 .
| Part Name | Theoretical switching temperature (°C) |
|---|---|
| BBa_K115029 | 42 |
| BBa_K115030 | 37 |
| BBa_K115031 | 37 |
| BBa_K115032 | 42 |
| BBa_K115033 | 37 |
| BBa_K115034 | 27 |
| BBa_K115035 | 32 |
| BBa_K115036 | 37 |
Setting up luciferase measurements
Luminescence of luciferase can be measured in two strains
The first two devices that were succesfully transformed were BBa_K115012 and BBa_K115034. First we wanted an indication whether it was possible to measure luciferase using these constructs, so an experiment was set up with these two strains. The exact protocol used can be found on the 19th of September of the lab notebook. In short, sonication and lysis buffer from the Promega Luciferase kit were used to lyse the cells and luciferase expression was normalized to OD600 of the original sample (before lysis). Furthermore, we cells were grown with and without arabinose induction as an inducible promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0080 BBa_R0080]) was used. Figure 3 is a graphical representation of the amount of luciferase measured for the two strains the conditions depicted.
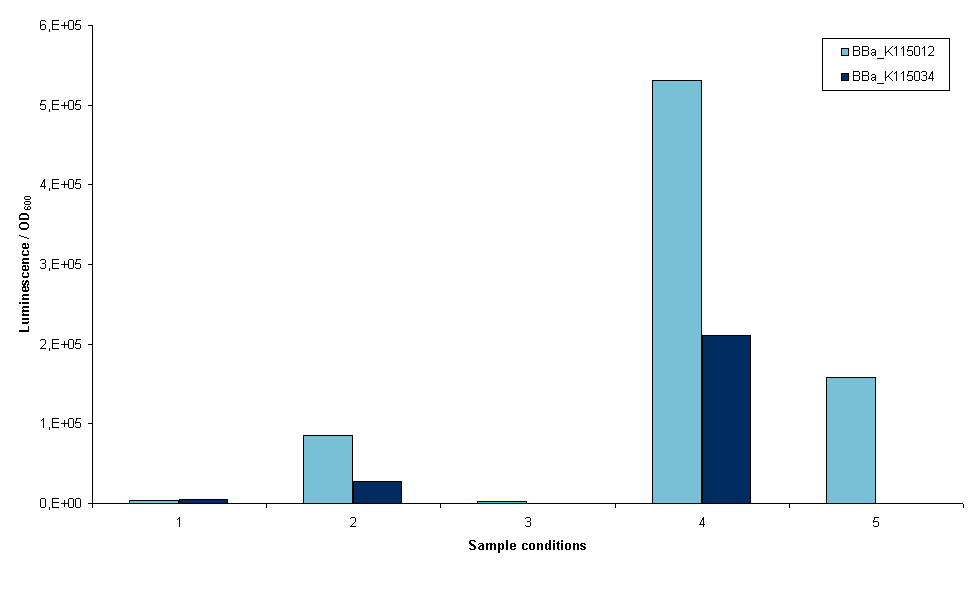
- 1. Induced growth overnight at room temperature, lysis by sonication
- 2. Induced growth overnight at 37ºC, lysis by sonication
- 3. Induced growth two times overnight at room temperature, lysis by sonication
- 4. Induced growth two times overnight at room temperature, lysis by Promega lysis buffer
- 5. Non-induced growth overnight at 37ºC, lysis by sonication (no induction at all, only BBa_K115012)
Several conclusions can be drawn from figure 3, although it has to be noted that this result is only one measurement. Furthermore, room temperature was not controlled. It may have fluctuated between 20 and 27ºC. The first conclusion is that we are able to measure luciferase with these constructs. This means that all parts used work correctly, including our ribosome binding site. The second conclusion is that a differential amount of luminescence is measured between strains BBa_K115012 and BBa_K115034, although in these results we cannot see a difference that is temperature dependent. This can be seen when the bars in conditions 1 and 2 are compared, BBa_K115012 gives a larger difference in luminescence from room temperature to 37ºC than BBa_K115034. The second conclusion is that luminescence measured when lysis is performed with the provided buffer is much higher (compare bars of condition 4 to the rest). The condition measured is not optimal as room temperature is used rather than 37ºC, but even now luminescence is a multitude higher than any of the sonicated samples. The reason for this is not clear: it could be that the lysis buffer lyses cells more efficiently, alternatively it could be that sonication denaturates a large part of the luciferase in the cell. Another conclusion is that the arabinose promoter does not work; a similar amount of luminescence was measured in samples where arabinose was added as compared to non-induced samples (figure 3, compare 5 to 1, 2, and 3).
Total protein content in stead of OD measurements
To compare the amount of luciferase expression measured, the total luminescence should be normalized. In the first experiment we normalized the luminescence to the OD600 in the culture. The OD600 is an indication for the amount of cells present. However, the amount of protein (total protein as well as luciferase) in the sample and thus the amount of luminescence is dependent on the lysis efficiency of the method used. Correcting total luminescence for total amount of protein would circumvent the lysis efficiency. After the first experiment it was decided that total protein content measurements would be conducted in the future. The OD600 will still be measured and lysis efficiency calculated. If a constant lysis efficiency (OD/protein content) is observed, we could still decide to normalize for OD600.
The protocol used for the next experiment measurements can be found here. In figure 4 the results are depicted per construct in luminescence per mg of total protein. A word of caution is in order when interpreting these results, as we found out when performing BC assays (performed according to [http://www.interchim.com/interchim/bio/produits_uptima/tech_sheet/FT-UP40840(BCA).pdf manufacturer's protocol]). The lysis buffer of the Promega luciferase assay kit reacts with copper residues used to determine protein content during the BC assay. Measuring 1x lysis buffer in the BC assay give a higher read-out than any of the samples in the standard calibration curve. The results presented here are obtained by diluting the samples with lysis buffer 1,000 times so their read-out fits within a normal calibration curve, without lysis buffer. Protein content used to normalize luminescence may differ a lot from the actual protein content in the samples, but protein contents could still be proportional.
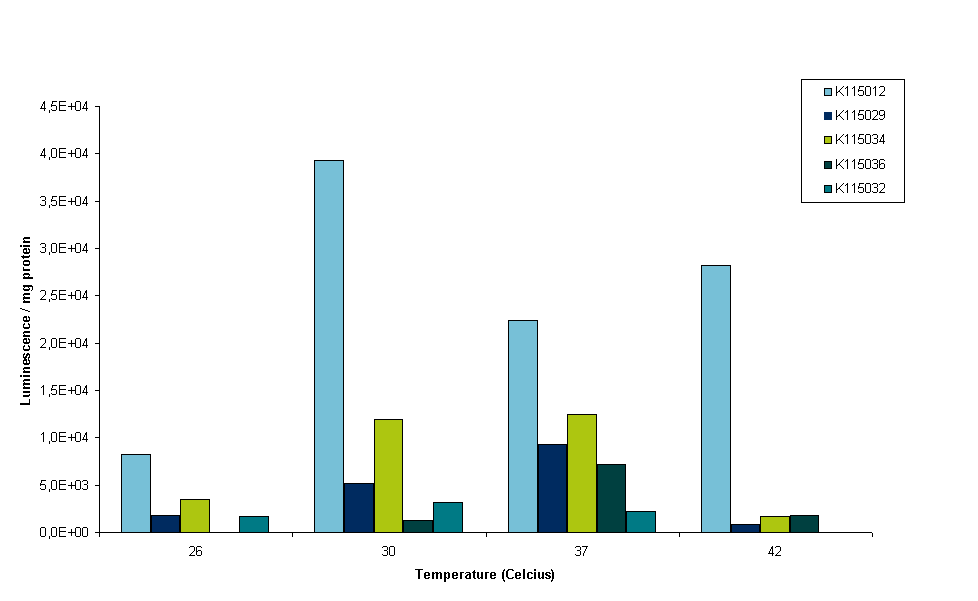
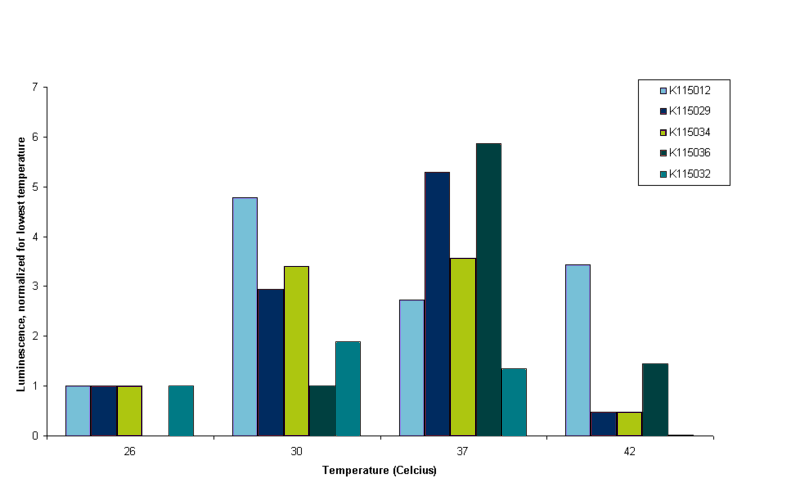
It can be seen in figure 4 that all constructs except BBa_K115012 show very little luminescence at 42ºC. OD readings were very low at this temperature, indicating that E. coli barely survives at that temperature. This is why we conclude that measurements at this temperature are not reliable. Although it is peculiar that BBa_K115012 does have a nice reading at 42ºC, especially since the 37ºC reading is lower than 30 and 42ºC (see figure 4, light blue bars). We think the 37ºC reading of BBa_K115012 was not a good reading, but to establish this we have to measure more samples in the same conditions and perform measurements at least in duplo to take into account biological variance. It was decided however to stop measuring at 42ºC. Further insight into these results is given by figure 5. Figure 5 gives an overview of the results presented in figure 4, but luminescence for each construct is normalized for the luminescence measured at the lowest temperature.
To interpret figure 5 it is important to note that BBa_K115012 is our control, non-temperature sensitive construct. Any 'switching' effect in the other constructs should present itself by an increase of luminescence greater than the standard increase that follows from a higher temperature. So we are looking for an increase in luminescence greater than that of BBa_K115012. From figure 5 it follows that this seems only the case for construct BBa_K115036 going from 30 to 37ºC (see figure 5, compare the bars for BBa_K115012 and BBa_K115036 at 30 and 37ºC). However, lack of a measurement at lower temperature for BBa_K115036, the fact that we performed the measurements only once and shaky protein content measurements make these results unreliable. For now the main conclusions drawn for this experiment is that we devised a protocol that allow us to measure luminescence from the constructs in parallel. The second conclusion is that cell lysis performed with lysis buffer provides us with yet another challenge as lysis buffer reacts with BC assay reagents. We could either try to get rid of the lysis buffer by performing a protein precipitation on the samples before protein content is measured (but not before luminescence is measured, as precipitation denatures proteins), or alternatively we could look for other ways to lyse the cells.
Protein precipitation protocols
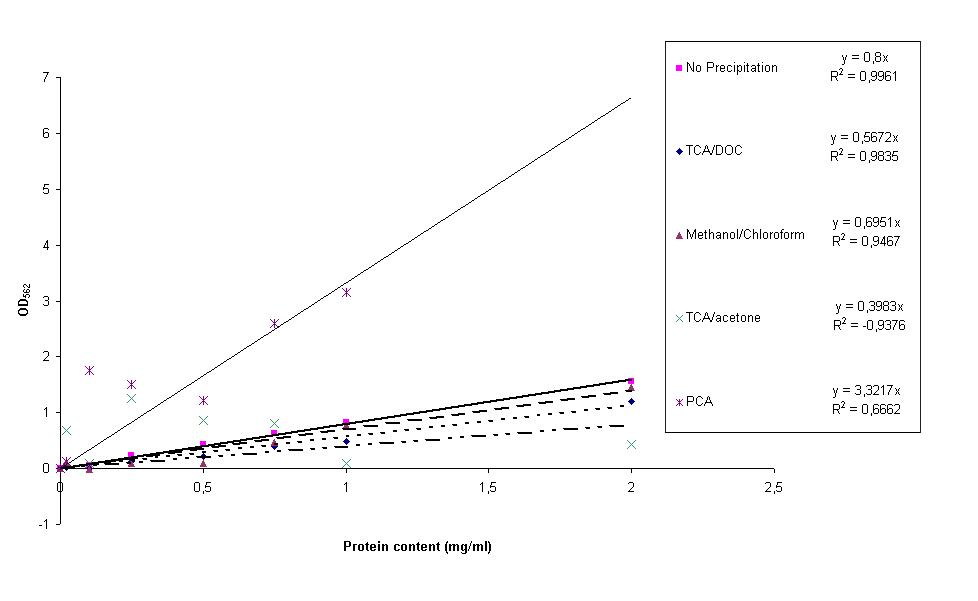
Four different protein precipitation protocols were conducted to obtain protein content measurements without the disturbance of lysis buffer. The four protocols can be found here, they are PCA, TCA/acetone, TCA/DOC and methanol/chloroform precipitation. For each of these precipitation methods four samples of constructs BBa_K115012, BBa_K115035 and BBa_K115036 and the calibration curve (bovine serum albumin in H2O) were resuspended in 1x lysis buffer. After OD562 was measured, standard calibration curves were made for all four precipitation protocols together with a 'normal' calibration curve for comparative reasons. The result can be seen in figure 6.
It is clear from figure 6 that the PCA and acetone/TCA calibration curves are really bad. This can be due to incomplete removal of the lysis buffer and/or differences in precipitation efficiency from sample to sample. The methanol/chloroform standard curve is already much better, but still not good enough for accurate experimentation. The TCA/DOC standard curve can be considered all right. However, a lot of the construct samples measured in this experiment for all precipitation methods gave back negative protein contents. A reason for this could be that protein precipitation in complex (cell extracts) samples takes longer than for simple (calibration curve) samples. During the experiments the shortest incubation periods possible were taken, this could be the reason we did not measure protein content. We started looking for other ways to lyse the cells because we were not able to measure protein content with any of these protocols.
Alternative cell lysis: bead beater, FastPrep and sonication
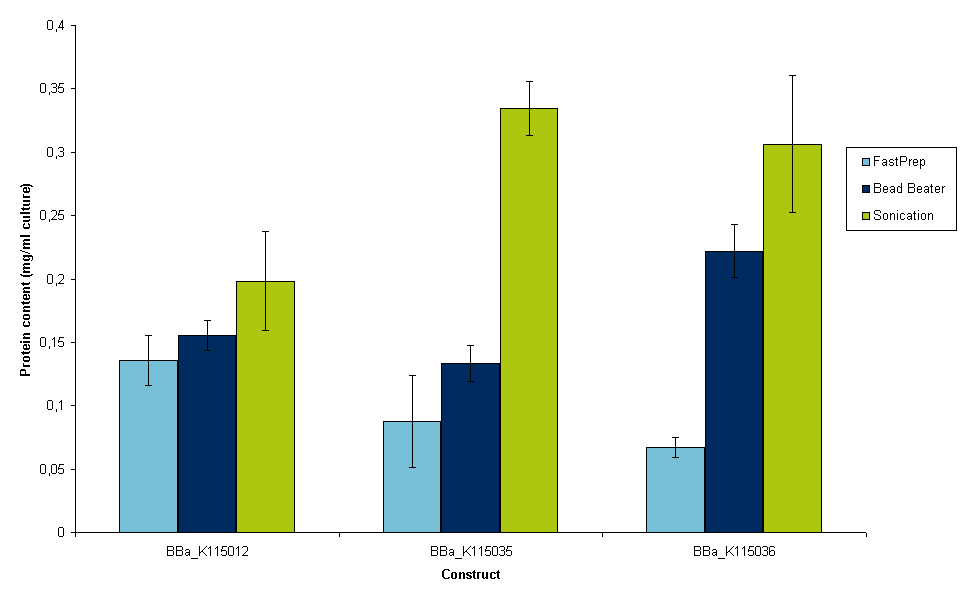
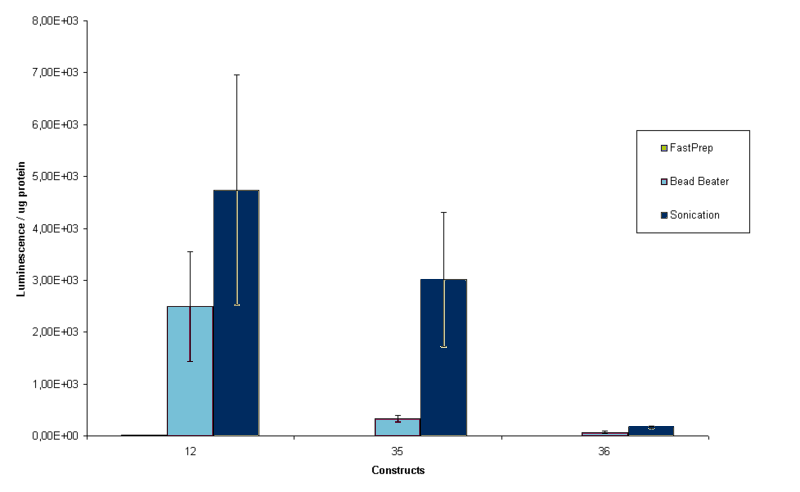
When protein precipitation methods did not work out, alternative methods of cell lysis were conducted. The protocols for cell lysis (including lysis buffer) can be found here. FastPrep and bead beater protocols were obtained from research groups at Delft UT, while experiments were conducted to determine the best sonication time for our sample. Results of that experiment can be found here. For three constructs, BBa_K115012, BBa_K115035 and BBa_K115036 protein contents were measured in duplo of 4 samples with similar OD600 to compare lysis of the cells. Results are shown in figure 7.
Sonication is the most time consuming lysis method, but it also gives the best result according to figure 7. Although succesful alternative cell lysis protocols were set up, we stil needed to know whether these new lysis methods kept at least part of the luciferase in its native state. To measure this, luciferase measurements were conducted on the samples that were used to make figure 7. The results of this measurement are shown in figure 8.
Figure 8 confirms that sonication is the best way to go for the luciferase measurements. In the construct BBa_K115012 measurement the difference between bead beater and sonication is not significant, but for the other two constructs it is. FastPrep did not yield any luminescence, probably because the FastPrep denaturates protein, at least at the intensity and time we used. The final protocol we settled on for the project can be found here.
Luciferase Measurements
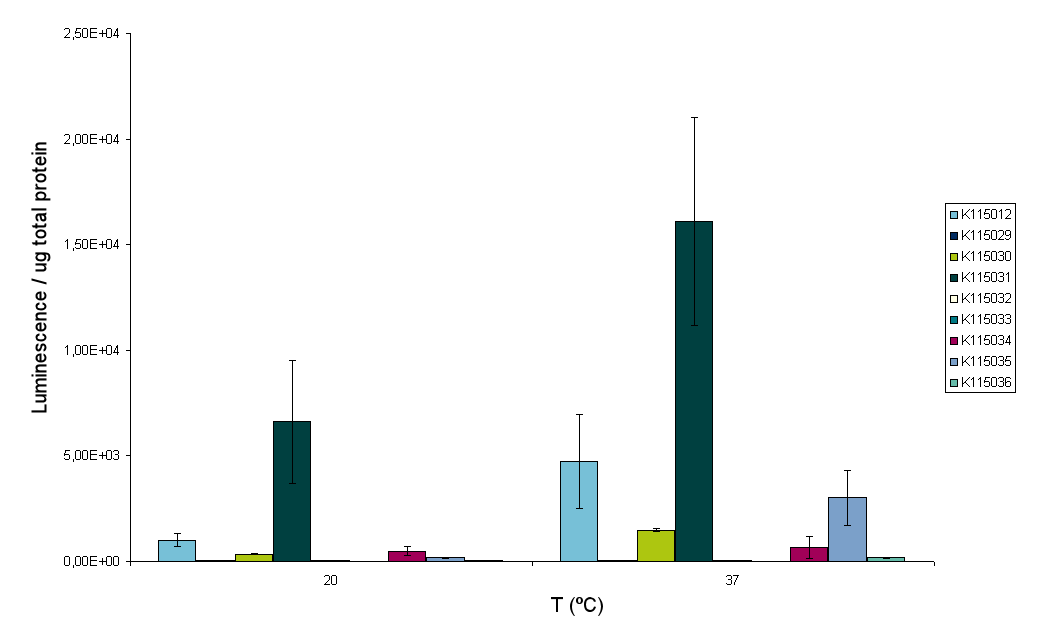
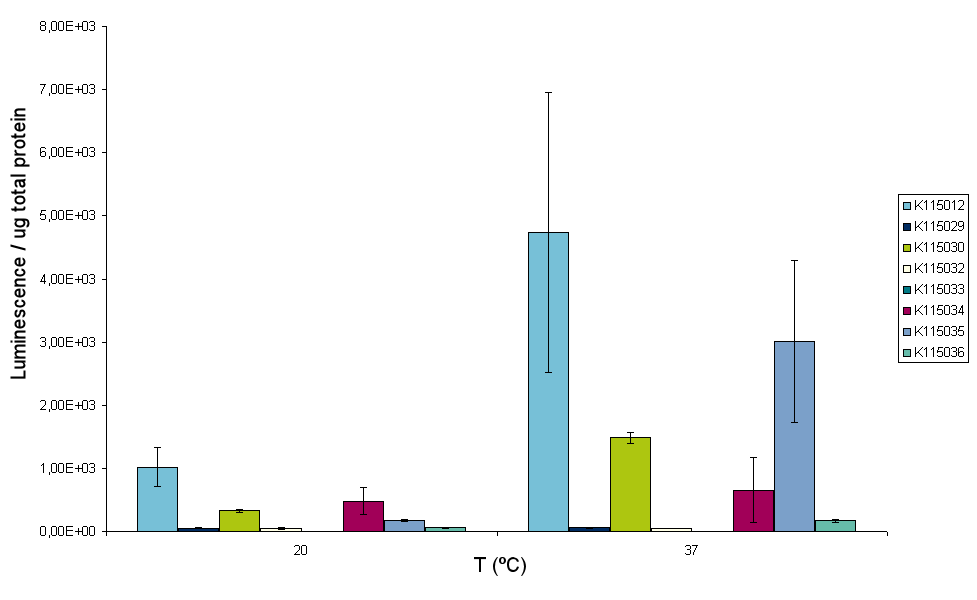
Luciferase measurements on all constructs and four different temperatures were conducted according to the protocol that was deviced before. However, while performing the experiment the sonicator broke down. At the time we already sonicated samples of two temperatures, 20 and 37ºC. But unfortunately all samples of 25 and 30ºC were lost for the measurement. The results for the 20 and 37ºC measurements are depicted in figure 9A and B.
From these figures at least two conclusions can be drawn. Firstly, the luminescence reading of BBa_K115031 is very high compared to the other constructs. Secondly, BBa_K115033 does not work at all. This could be due to incorrect assembly of the construct. For further insight in the experiment figure 10 was made. Figure 10 shows the fold increasement of luminescence from 20ºC to 37ºC for all constructs.
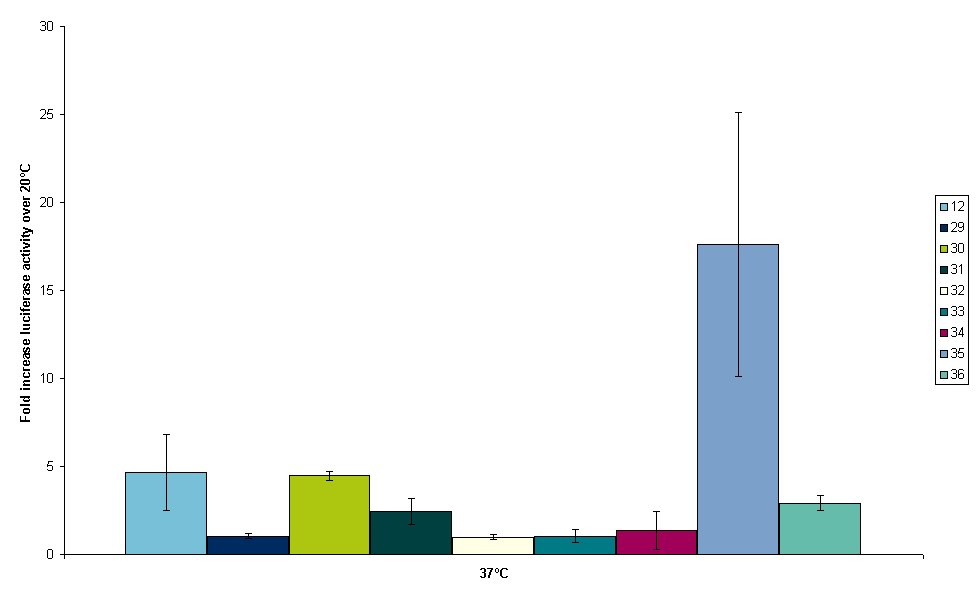
From figure 10 it is clear that we have one construct, BBa_K115035, that has an increase of luminescence that is significantly higher than the increase of BBa_K115012. Due to time constraints, it was decided that we conduct one more experiment with only BBa_K115012 and BBa_K115035 as this construct has the highest probability of bearing a working RNA thermometer. One more experiment was conducted at 30ºC with only the control non-temperature sensitive strain BBa_K115012 and BBa_K115035 (that is supposed to switch at 32ºC). Figure 11 shows the results of measurements at 20 and 37ºC that were conducted before and 30ºC.
The results obtained for 30ºC are consistent with the findings at 20 and 37ºC. Luminescence and thus luciferase expression goes up from 20 to 30ºC for both constructs, but going from 30 to 37ºC the increase is clearly higher for BBa_K115035 than for BBa_K115012. BBa_K115035 is supposed to switch at 32ºC and according to these data it is not switched on at 30ºC. Finally, table 2 shows the fold increasement of luminescence for BBa_K115012 and BBa_K115035 at 30 and 37ºC. These numbers are normalized for the amount of luminescence of these strains at 20ºC, so at 20ºC the strains have index number 1.00.
| Temperature | BBa_K115012 | BBa_K115035 |
|---|---|---|
| 20ºC | 1.00 | 1.00 |
| 30ºC | 2.44 | 1.92 |
| 37ºC | 4.66 | 17.6 |
</div>
</div>
</div>
 "
"