Team:Bologna/UV radiation/
From 2008.igem.org
Rita.morini (Talk | contribs) (→Protocol used for UV induction) |
Rita.morini (Talk | contribs) (→Protocol used for UV induction) |
||
| Line 61: | Line 61: | ||
In order to show the correct functionality of our construction, induction by peroxide hydrogen was used, in fact a low concentration of H_2 O_2 (1-3mM) results in SOS gene induction in wild-type cells [Imlay and Linn, 1987; Goerlich et alt. 1989]. | In order to show the correct functionality of our construction, induction by peroxide hydrogen was used, in fact a low concentration of H_2 O_2 (1-3mM) results in SOS gene induction in wild-type cells [Imlay and Linn, 1987; Goerlich et alt. 1989]. | ||
The induction of SOS by H_2 O_2 is an important event, since RecA and recBC mutant cells are very sensitive to H_2 O_2 treatment probably due to the lack of recombinational repair necessary for the H_2 O_2 – induced lesions [Imlay and Linn, 1987]. | The induction of SOS by H_2 O_2 is an important event, since RecA and recBC mutant cells are very sensitive to H_2 O_2 treatment probably due to the lack of recombinational repair necessary for the H_2 O_2 – induced lesions [Imlay and Linn, 1987]. | ||
| - | <br> | + | <br><br><br> |
[[Image:schema.jpg|right]] | [[Image:schema.jpg|right]] | ||
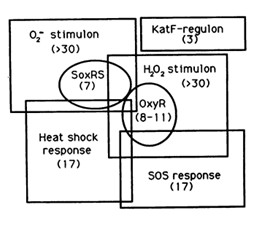
The reaction of hydrogen peroxide with transition metals imposes on cells an oxidative stress condition that can result in damage to cell components such as proteins, lipids and principally to DNA. Escherichia Coli cells are able to deal with these adverse events via DNA repair mechanisms or OxyR and SosRS anti-oxidant inducible pathways which are elicited by cells to avoid the introduction of oxidative lesions by hydrogen peroxide. | The reaction of hydrogen peroxide with transition metals imposes on cells an oxidative stress condition that can result in damage to cell components such as proteins, lipids and principally to DNA. Escherichia Coli cells are able to deal with these adverse events via DNA repair mechanisms or OxyR and SosRS anti-oxidant inducible pathways which are elicited by cells to avoid the introduction of oxidative lesions by hydrogen peroxide. | ||
Among the systems the OxyR gene interacts with, there is the SOS response. | Among the systems the OxyR gene interacts with, there is the SOS response. | ||
Revision as of 11:03, 28 October 2008
| HOME | PROJECT | TEAM | SOFTWARE | MODELING | WET LAB | SUBMITTED PARTS | BIOSAFETY AND PROTOCOLS |
|---|
UV Spectrum
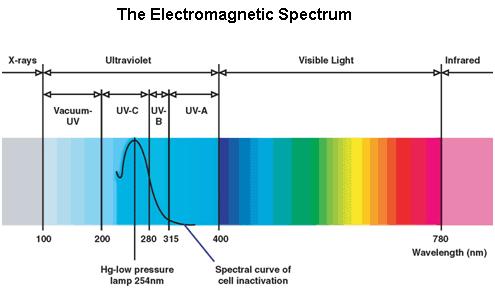
Ultraviolet is that part of electromagnetic radiation bounded by the lower wavelength extreme of the visible spectrum and the upper end of the X-ray radiation band. The spectral range of ultraviolet radiation is, by definition, between 100 and 400 nm and is invisible to human eyes. The UV spectrum is subdivided into three bands: UV-A (long-wave) from 315 to 400 nm, UV-B (medium-wave) from 280 to 315 nm, UV-C (short-wave) from 100 to 280 nm. A strong germicidal effect is provided by the radiation in the short-wave UV-C band.
E.coli SOS System
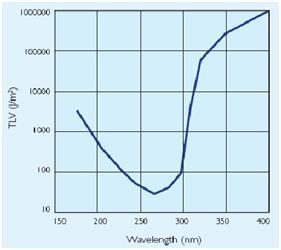
The maximum UV germicidal effect coincides with the peak absorbance of DNA (near 260nm) due to the dimerization of two adjacent thymines. That can be seen in the Fig.1 where is showed the living population of bacteria after irradiation.
E.Coli cells have a system that recovery DNA damage when it occurs and the best studied transcriptional response to DNA damage is the SOS response [Friedberg et al., 1995; Walker, 1996].
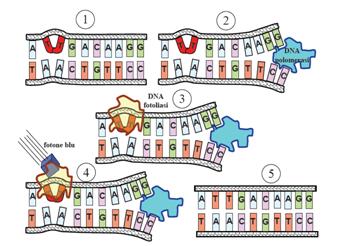
This systems can be divided into two class: the SOS Photoreactivation repair and the SOS triggered by RecA protein. The first uses the photolyase, a poorly expressed enzyme(encoded by genes phrA and phrB) which binds the pyrimidine dimers and uses the blue light to split them apart as showed in Fig.2.
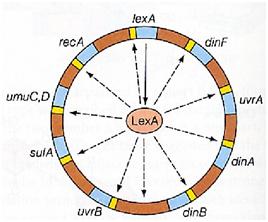
Instead single stranded DNA produced by several DNA-damaging agents can be bound by RecA protein, resulting in conversion of this protein to its activated form. The RecA repair system doesn’t need light and Lexa protein controls the expression of 43 genes [Courcelle et al. (2001)] that cooperate together to repair the extensive genetic damage. RecA and LexA proteins play an important rule on the regulatory of SOS Recombination System.
A LexA binding site is present in all the SOS promoters genes' and it works as a repressor of SOS system. In presence of DNA damage (DNA Single Strains) RecA becomes active and interacts with LexA protein , the repressor of the SOS genes [Wagner et al., 1999]. This interaction triggers the autocatalytic cleavage of LexA and consequent destruction of its ability to function as a repressor, which results in the derepression of SOS genes (Mustard and Little, 2000; Fernandez De Henestrosa et al., 2000).
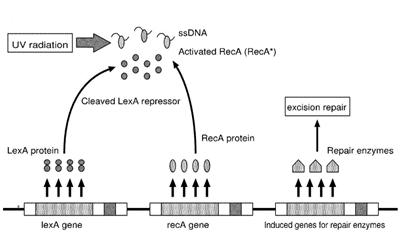
When the damage is repaired, DNA single strains are not present in the cell and the RecA protein no longer promotes the auto-cleavage of the LexA which is restored to its initial repression level.
Protocol used for UV induction
The plasmid with our construct has been transformed in DH5alfa bacteria by standard protocol and one colony from the plate has been picked and cultured overnight in 5ml LB medium broth with ampicilline.
The day after the culture has been diluted in 5ml LB and antibiotic in order to have 0.1 starting OD bacteria culture and let it grown for one hour; after that the culture has been divided in five falcons with 1ml of bacteria, ready to be induced. Using 1ml of culture is a choice done in order to have a thickness as thick as possible to perform an irradiation as uniform as possible .
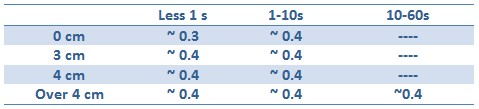
Tests with difference distances from the lamp with different exposition times were done in order to respect the maximum lethal UV dose of bacteria and avoid mutagenesis factors in the cell. The following table illustrates the tests setup used:
After induction by UV the samples were kept in dark by silver paper to increase the RecA and LexA answer and let grown for 2h. The OD sample is measured and the sample transferred in a 1ml eppendorf spinned at 6000-8000rpm for three minutes and pellet collected in order to measure fluorescence level by microscope and Bacteria Visual Fluo Software.
Unfortunately, using Falcon tube and not the correct time/distance induction the experimental tests showed weak GFP expression using the construct with straighter promoter. It could be depended by the low sensibility of SOS system with our setup tests and not uniform irradiation of the sample. In Fig.3 is possible to see a leak answer by bacteria stimulated by UV at the distance of 4cm with one second time exposure.
In order to show the correct functionality of our construction, induction by peroxide hydrogen was used, in fact a low concentration of H_2 O_2 (1-3mM) results in SOS gene induction in wild-type cells [Imlay and Linn, 1987; Goerlich et alt. 1989].
The induction of SOS by H_2 O_2 is an important event, since RecA and recBC mutant cells are very sensitive to H_2 O_2 treatment probably due to the lack of recombinational repair necessary for the H_2 O_2 – induced lesions [Imlay and Linn, 1987].
The reaction of hydrogen peroxide with transition metals imposes on cells an oxidative stress condition that can result in damage to cell components such as proteins, lipids and principally to DNA. Escherichia Coli cells are able to deal with these adverse events via DNA repair mechanisms or OxyR and SosRS anti-oxidant inducible pathways which are elicited by cells to avoid the introduction of oxidative lesions by hydrogen peroxide. Among the systems the OxyR gene interacts with, there is the SOS response.
 "
"