Team:Cambridge/Bacillus/Lab Work
From 2008.igem.org
July 22nd
We have 4 tubes from last year. These strains are frozen in glycerol.
- PNZ8901 plasmid in E.coli MC1061 Strain
- Chloroamphenicol resistant
- "sure" vector
- does not integrate
- B. subtilis strain 1A1
- No resistance
- Same as strain 168 but tryptophan deficient
- E.coli strain MC1061, no plasmid
- PP182 plasmid in E.coli strain DH5alpha
- Ampicillin resistance
- integrates
Checking antibiotic resistance
Purpose : Grow each of them on a plate to test their antibiotic resistance
Protocol
- Warm up frozen tubes
- Take 15uL of each and put it on plates without antibiotic
- Incubate at 37°C
July 23rd
- Take one colony from each of the plates grown from the tubes yesterday and plated them again on LA without antibiotics
1/ Purpose Check antibiotic resistance of our different strains
| Strain | Plasmid | Extracted from | Antibiotic use | Concentration of antibiotic |
|---|---|---|---|---|
| DH5alpha | PP182 | tube | Amp | 100 |
| MC1061 | NONE | tube | Cm | 35 |
| MC1061 | PNZ8901 | tube | Cm | 35 |
| DH5alpha | PP182 | plate | Amp | 100 |
| MC1061 | NONE | plate | Cm | 35 |
| MC1061 | PNZ8901 | plate | Cm | 35 |
2/ Purpose : Find the right concentration of antibiotic so that B. subtilis survive
- Grow 15uL of B. 1A1 (frozen tube) in 5mL of LB
- Incubate at 37°C
3/ Purpose : Grow plasmids in TOP10, transformation
4 plasmids :
- I746000
- I746100
- I746101
- I746001
1 control : PUC19
- Add 20uL of TOP10 competent cells and 0.5uL of Plasmid in an eppendorf
- 30min on ice
- Heat shock : 60s at 42°C
- 2min on ice
- Add 89.5uL of SOC
- 60min at 37°C
- Put the mix on plate with ampicillin resistance
- Incubate at 37°C
July 24th
- Test of antibiotic resistances of strains of last year
| Strain | Plasmid | Extracted from | Antibiotic use | Observations | Conclusion |
|---|---|---|---|---|---|
| DH5alpha | PP182 | tube | Amp | Many colonies | Amp Resistance OK |
| DH5alpha | PP182 | plate | Amp | Many colonies | Idem |
| MC1061 | NONE | tube | Cm | Nothing | As expecting, no Cm Resist. |
| MC1061 | NONE | plate | Cm | Nothing | Idem |
| MC1061 | PNZ8901 | plate | Cm | Maybe a few colonies | Contamination?? |
| MC1061 | PNZ8901 | tube | Cm | No colonies | no Cm Resist. |
- Transformation of E.coli with different plasmids from last year
| Strain | Inserted plasmid | Antibiotic | Observation | Conclusion |
|---|---|---|---|---|
| E.coli | I746000 | Amp | No colonies | Antibiotic resistance unknown, no Amp Resist., Pb : no terminator |
| E.coli | I746100 | Amp | No colonies | Antibiotic resistance unknown, no Amp Resist., Pb : no terminator |
| E.coli | I746001 | Amp | Many colonies | Transformation OK |
| E.coli | I746101 | Amp | Many colonies | Transformation OK |
Antibiotic test for Bacillus resistance
We want to find the lowest concentration of antibiotic which kills Bacillus.
Dilution of Amp. [ 1:1000 means 1 part stock to 1000 part Sterile Distilled Water ]
| Concentration (μg/mL) | 100 | 75 | 50 | 25 | 10 |
|---|---|---|---|---|---|
| 100 mg/mL Stock | 1:1000 | 3:4000 | 1:2000 | 1:4000 | 1:10000 |
Dilution of Cm. [ 1:1000 means 1 part stock to 1000 part Sterile Distilled Water ]
| Concentration (μg/mL) | 35 | 25 | 15 | 10 | 5 |
|---|---|---|---|---|---|
| 100 mg/mL Stock | 1:1000 | 1:1400 | 3:7000 | 1:3500 | 1:7000 |
- Add Disks in antibiotic solution for 5 mins [ Protocol to sterilize tweezers : Wipe with Kimwipes, Ethanol, Flame ]
- Melt Soft Agar
- 3mL SA + 10μL Cells 1A1 from LB prepared on 23/7/08
- Pour SA+Cells over blank hard agar plates
- put disks with different concentration of Amp/Cm above SA
- Incubate at 37°C
Results [Plates were prepared before lunch, at 5pm there were visible growth]
-Amp Plates: Huge rings of no growth around 100, 75, 50, 25, and 10.
-Cm PLates: Tiny rings of no growth around 5, 10, 15 and 25. Small ring of no growth around 35 but not well defined. (No clear zones)
Salts for making Bacillus Competent
10x Medium A Base
- Yeast Extract 10g
- Casamino Acid 2g
- add Distilled water to 900mL
-Aliquot into 5 different bottles. [180mL each]
10x Bacillus Salts
- NH4)2SO4 20g
- K2PO4 anhydrous 139.66g
- KH2PO4 60g
- Tri-Sodium Citrate 10g
- MgSO4.7H2O 2g
- Add Distilled water to 1000mL
-Aliguot into 5 different bottles. [200mL each]
Medium B
- Preparing 50mM CaCl2.2H2O
- CaCl2.2H2O 1.470g
- Add Distilled water to 20mL [Final conc. 500mM]
- Take 10mL of 500mM
- Add 90mL of distilled water [Final conc. 50mM]
- Preparing 50mM CaCl2.2H2O
- Preparing 250nM MgCl2.6H2O
- MgCl2.6H2O 0.508g
- Add Distilled water to 10mL [Final conc. 250mM]
- Take 1mL of 250mM
- Add 99mL of Distilled water [Final conc. 250μM]
- Take 1mL of 250μM
- Add 99mL of Distilled water [Final conc. 250nM]
- Preparing 250nM MgCl2.6H2O
- Add 100mL of 50mM CaCl2.2H2O
- Add 100mL of 250nM MgCl2.6H2O
-Total volume 200mL
NOTE: To complete Medium B, Take 0.2mL of this solution
July 25th
Results from Yesterday
- Antibiotic test
Results for Amp
Even with a concentration of 10μg/mL, Amp kills Bacillus. Since we want to know which is the lowest concentration of Amp which kills B.S, we are going to test with some lower concentrations.
Results for Cm
Even with a concentration of 35μg/mL, ther is not a clear area around the antibiotic disk. So we have to test some higher concentration of Cm.
Wet Work
- New antibiotic tests
Dilution of Amp
| Concentration (μg/mL) | 10 | 7.5 | 5 | 2.5 | 1 |
|---|---|---|---|---|---|
| 10 μg/mL Stock (from yesterday) | 1:1 | 3:4 | 1:2 | 1:4 | 1:10 |
Dilution of Cm
| Concentration (μg/mL) | 35 | 37.5 | 40 | 42.5 | 45 |
|---|---|---|---|---|---|
| 35 μg/mL Stock (from yesterday) | 1:1 | 5:6 (from 45μg/mL) | 1:875 | 17:18 (from 45μg/mL) | 9:7000 |
- Melt Soft Agar - 3mL SA + 10μL Cells 1A1 from LB prepared on 23/7/08 - Pour SA+Cells over blank hard agar plates - Put 5 blank disks on each agar plate - Add 40μL of antibiotic on each disk - Incubate at 37°C
Results
Nothing! 1A1 cells were kept in the freedge! B.S. can not be kept in the fridge, low temperatures kill them!
July 28th
- Results of antibiotic plates from yesterday
For Cm, 35μg/mL should be enough to kill B.S.
For Amp, nothing can be concluded!
Wet Work
- Check plasmid ppL82
We had 2 samples of ppL82 in DH5α in LB solution (~4mL), one from the frozen glycerol tube and one from a colony picked on a plate. Normally plasmid and cells should not be kept in freezer. So, we want to extract this plasmid, check its size and then keep it in the fridge. We do that for the 2 different samples.
- Plasmid Miniprep (standard protocol)
- Test the concentration of DNA in each tube
- ppL82 plate : 89.8ng/mL
- ppL82 tube : 71ng/mL
- Preparation of different stocks of strains and plasmids
PNZ8901
- Plate a single colony (from 23/07/08) on a Cm plate
- Incubate at 37°C
- Grow the entire frozen glycerol tube in 20mL of LB without antibiotic to check if this stock is still good (we will check the plasmid on a gel tomorrow)
- Incubate at 37°C
MC1061
-Pick e single colony of MC1061 (from 22/07/08) and put it in 10mL of LB (to make glycerol stocks)
-Incubate at 37°C
1A1
- Add 100μL of LB+1A (mix of friday) and 10mL of LB
- Incubate at 37°C
- Make B.S. 1A1 competent and transform them
- Prepare Medium A
Add 81mL of SDW, 10mL of 10X Medium A base and 9mL of 10X Bacillus salts
- Make B.S. 1A1 competent
- In 10mL of medium A and add about 10 colonies of B.S.
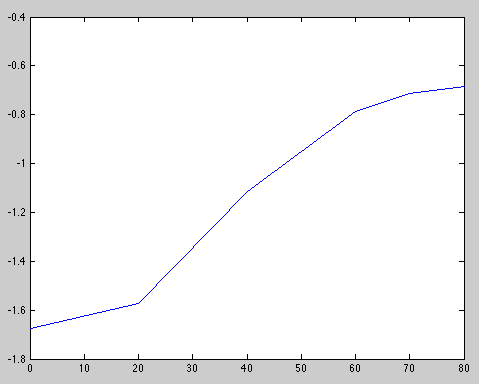
- Check the OD650, you should have an OD between 0.1 and 0.2. t0 for OD = 0.1876
- Check the OD650 every 20min and plot OD against time on semi-log paper. When the point at the culture leaves log growth, it is ok
| Time (min) | 0 | 20 | 40 | 60 | 70 | 80 |
|---|---|---|---|---|---|---|
| OD650 | 0.1876 | 0.2074 | 0.3282 | 0.4545 | 0.4895 | 0.5040 |
At, t0 = 70min, log growth seems to stop.
- Incubate at 37°C 90min after t0
- Warn 10 Ependorf tubes with 0.45mL of Medium B
- Add 50μL of culture in each tube of Medium B at t90
- Incubate the diluted culture at 37°C with vigorous aeration for 90min
- Storage : we want to store 7 tubes
6 tubes in the freezer (with 60μL of glycerol)
1 tube on the bench
- Transformation
- We make ppL82 from plate, ppL82 from tube, and a control
- Add 0.5μg of DNA (15μL)
- Incubate at 37°C for 30min
- Plate on Cm plates
July 29th
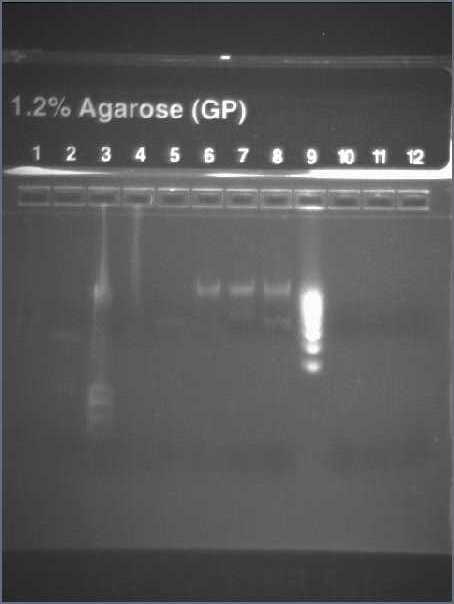
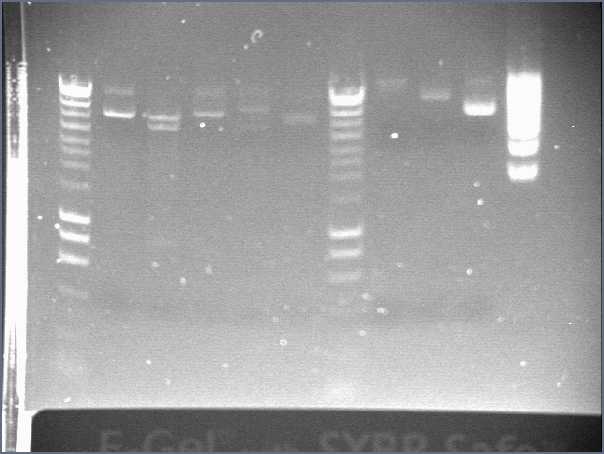
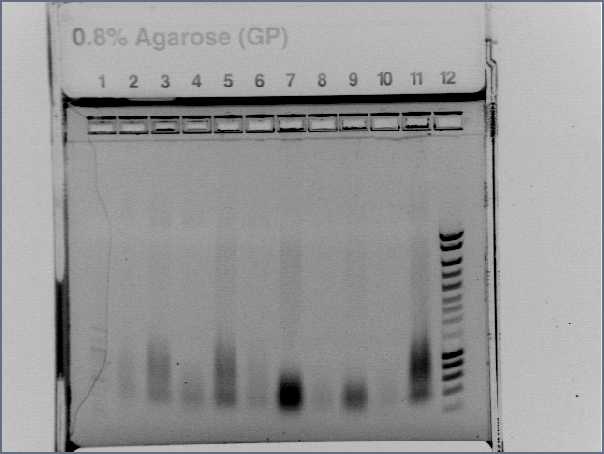
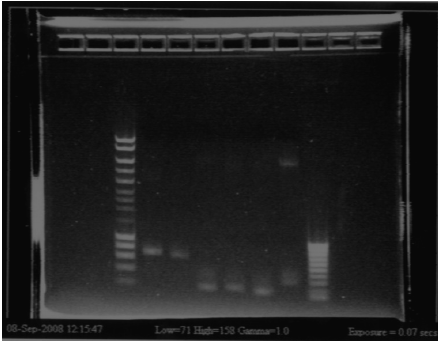
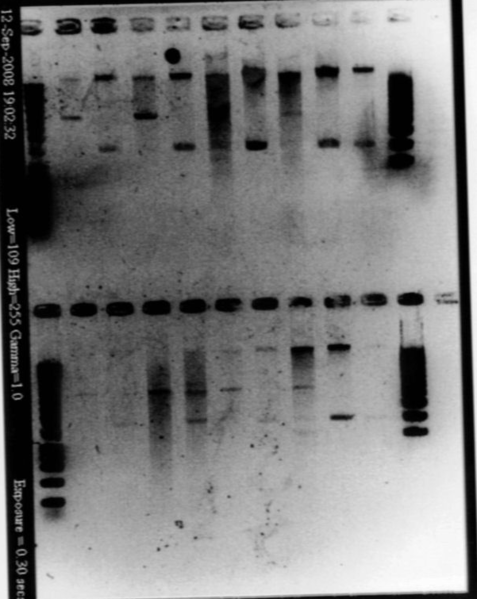
- Result from the gel (29/07/2008)
- Lane8 : ppL82 (plate)
- Lane9 : ppL82 (tube)
- Lane 10 : I746001
- Lane 11 : I746101
- Lane 12 : hyperladderI
The ladder seems to be wrong. So it was really difficult to ckeck the size of our plasmid ppL82. To make, that, we assume that the size of our biobricks was ok, and we estimate the size of our plasmid. The plasmid ppL82 seems to have the right size, but we will have to check again to be able to use a ladder.
- Competence of B.S. kept on the bench
We observed B.S. with a microscope. Problem, all B.S. seems to be dead!
Possible reasons of this problem :
- Problem with the vector (it could be a wrong vector, we are not really sure)
- Quantity of DNA, we added 0.5μg. The protocol was 1μg, but normally it should be ok.
- B.S. may not be competent (we have to test motility with microscope)
- Cells could be dead : replate them
- Try to add tryptophan in the medium
Probable reason : we forgot to dilute the medium B, that's why it did not work
- Results from transformation plates (B.S.)
There are 2 colonies on a plate, but it does not look like Bacillus. Moreover, there is also a colony on our negative control plate. SO it must be contamination (resistant to CM!). We will do the transformation again.
Wet Work
- Prepare medium for transformation of B.S.
- Preparation of medium B: 10mL of medium A and 0.2mL of 50mMCaCl22(H2O) + 250mMgCl26(H2O)
- Preparation of medium A with tryptophan : 81mL of SDW, 9mL of 10X Bacillus salts, 10mL of 10X Medium A base and 0.1mL of Tryptophan (11mg/mL)
- Check plasmid PNZ8901
- Plasmid miniprep (same protocol with 60μL of elution buffer)
- Digest : with PstI and SalI from Biolabs, and Buffer 3 (add 15μL of DNA)
- Gel (17μL of sample and 3μL of dye)
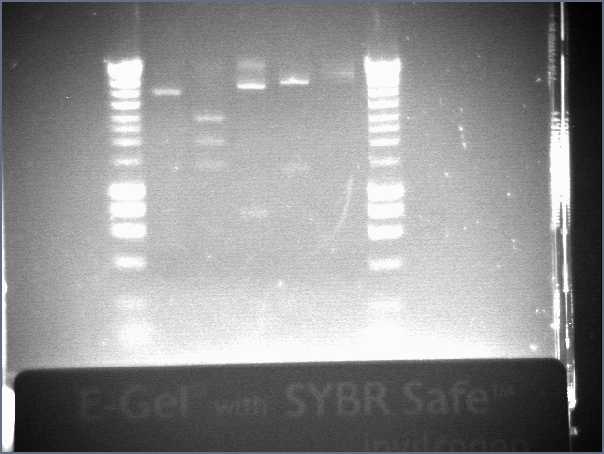
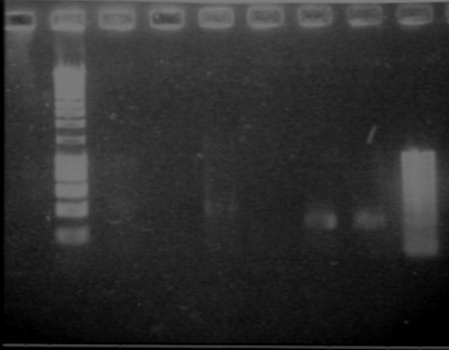
Results from the gel
- Lane9 : PNZ8901
- Lane10 : HyperladderI
The sizes we expected were about 1100kb and 2100kb. The sizes should be ok!
July 30th
- Transforming Bacillus Subtilis with medium A and medium A + tryptophan
Medium A
| Time (min) | 0 | 20 | 40 | 60 | 73 | 85 | 95 | 105 |
|---|---|---|---|---|---|---|---|---|
| OD650 | 0.1394 | 0.1370 | 0.1660 | 0.2304 | 0.2778 | 0.3138 | 0.3373 | 0.3583 |
For this medium, the curb stop to increase logarithmically at 100min.
Medium A + tryptophan
| Time (min) | 0 | 20 | 40 | 60 | 73 | 85 | 95 |
|---|---|---|---|---|---|---|---|
| OD650 | 0.1493 | 0.1636 | 0.2080 | 0.2909 | 0.3512 | 0.4002 | 0.4165 |
For this medium, the curb stop to increase logarithmically at 90min.
- Incubate 90min at 37°C by shaking
- Prepare 7 tubes for medium A and 7 tubes for medium A + tryptophan : add 0.45mL of prewarmed medium B and 0.05mL of culture and incubate 90min at 37°C by shaking.
- Transform B.S. by adding DNA and incubate 30min at 37°C
We have one transformation with ppL82 (add 7.5μL of DNA), one with PNZ8901 (add 10μL of DNA) and one negative control without DNA.
- Plate 500μL on Cm plate (35μg/mL)
- For the remaining tubes, in one, add 60μL of glycerol and keep it in the freezer and keep the other one on the bench (for each medium)
- Checking our big stock of biobricks and PNZ plasmid
We did big culture of biobricks and PNZ8901 plasmid to be able to make stocks. We want to check them before making stocks.
- Plamsid miniprep (for I746001, I746101 and PNZ8901 from big flasks
- Measure DNA concentration in our samples to decide the volume of DNA we have to add in our preparation
| 260/280 | DNA concentration (ng/nl) | |
|---|---|---|
| I746001 | 1.79 | 50.5 |
| I746101 | 1.87 | 54.3 |
| PNZ8901 | 1.84 | 87 |
- Digest
| For I746001 and I746101 | For PNZ8901 plasmid |
|---|---|
| 14μL of SDW | 14μL of SDW |
| 2μL of 10X Fast Digest Buffer | 2μL of Buffer 3 (Biolabs) |
| 2μL of DNA of biobricks | 2μL of DNA of PNZ8901 |
| 1μL of EcoRI | 1μL of PstI |
| 1μL of SpeI | 1μL of SalI |
- Gel
- Results from this gel
- Lane 9 : I746001
- Lane 10 : I746101
- Lane 11 : PNZ8901
- Lane 12 : HyperladderI
Everthing is really too big! There is a problem, either dimerization, either contamination, either a problem in our work. So we are going to run a new gel.
- New gel to check
- Miniprep plasmid from growth bottles (I746001, I746101 and PNZ8901); from plates (I746001, I746101 and PNZ8901).
- Do single digest for PNZ8901 (one with PstI, one with SalI)
- Run a gel with : PNZ8901 from friday, PNZ8901 digest with PstI, PNZ8901 digest with SalI, 3 samples from growth bottles, 3 samples from plates (to check the size of the uncut vectors)
- Result from this second gel
The ladders are really bad! But the size of our biobricks and plasmid are too big! So we can not trust these big cultures. They is a problem. With tests from the first week, we know that biobricks are right, so we are going to grow new cultures from these first cultures, to make stocks.
Concerning the vectors, they are from last year, so we are not sure of what they are. Since we ordered new well defined vectors (we should receive them on friday), we will use them in the next steps to be sure of our work.
August 1st
- Grow received vectors
| Vectors | AmpR (μg/μL) | CmR (μg/μL) | KanR (μg/μL) |
|---|---|---|---|
| ECE112 | 100 | 0 | 0 |
| ECE147 | 50 | 0 | 0 |
| ECE149 | 50 | 0 | 0 |
| ECE150 | 50 | 0 | 0 |
| ECE151 | 50 | 0 | 0 |
| ECE153 | 100 | 0 | 0 |
| ECE162 | 100 | 0 | 0 |
| ECE165 | 100 | 10 | 0 |
| ECE166 | 100 | 10 | 0 |
| ECE171 | 50 | 0 | 10 |
| ECE172 | 100 | 10 | 0 |
| ECE176 | 100 | 5 | 0 |
- Preparation of Agar plates
We have bottles of 200mL.
| Type | Amp (μL) | Cm (μL) | Kan (μL) |
|---|---|---|---|
| Amp100 | 200 | 0 | 0 |
| Amp50 | 100 | 0 | 0 |
| Amp100 + Cm10 | 200 | 57.1 | 0 |
| Amp100 + Cm5 | 200 | 28.6 | 0 |
| Amp50 + Kan10 | 50 | 0 | 80 |
- Preparation of LB tubes
We prepare 10mL of LB in which we add a single colony.
| Type | Amp (μL) | Cm (μL) | Kan (μL) |
|---|---|---|---|
| Amp100 | 10 | 0 | 0 |
| Amp50 | 5 | 0 | 0 |
| Amp100 + Cm10 | 10 | 2.9 | 0 |
| Amp100 + Cm5 | 10 | 1.4 | 0 |
| Amp50 + Kan10 | 5 | 0 | 4 |
August 2nd
Yesterday's Results
- All Plates grew!
- ECE 172 seemed to have trouble growing on the plate (no single colonies, just one big lump at the start of the streak)
- ECE 150 grew too well! (possibly hard to pick out single colony)
- Tubes:
- ECE 176, 172, 153 seem to be quite clear!!
- ECE 150, 151 had strange floating 'colonies' in the tube
- All other tubes were quite cloudy
Lab Work
Fridged all plates except ECE 172 ==> Benched.
All tubes palleted and placed in freezer although ECE 176, 172, 153 doesn't seem to have any pallets
All red plates does not seem to have any extra growth despite the air conditioning had been turned off when I entered the lab this afternoon.
August 4th
Wet Work
- Check vectors
- for each vector, plasmid miniprep from frozen pellets (step 7 only once, with 60μL of Elution Buffer for ECE112, ECE147, ECE149, ECE 150 and only 30μL for the others)
- Single digest (15μL of SDW, 2μL of Buffer, 2μL of DNA, 1μL of enzyme, then incubate 10min at 37°C and 30 min for the one using HindIII with EcoRI buffer)
- Double digest (mix 7.5μL of each single digest, and then incubate 10min (or 30min for EcoRI + HindIII and EcoRI buffer) at 37°C, then heat shock 5min at 80°C
| Vectors | Enzyme 1 | Enzyme 2 | Buffer | Entire size | Size of part1 | Size of part2 |
|---|---|---|---|---|---|---|
| ECE112 | XbaI | EcoRI | Fast Digest | 10156 | 6900 | 3295 |
| ECE147 | EcoRI | HindIII | EcoRI | 5482 | 4913 | 600 |
| ECE149 | SpeI | PstI | Fast Digest | 6192 | 4919 | 1300 |
| ECE150 | SpeI | HimdIII | Buffer 2 | 6166 | 5300 | 778 |
| ECE151 | SpeI | EcoRI | Fast Digest | 6166 | 4825 | 1300 |
| ECE153 | SalI | Buffer 3 | ||||
| ECE162 | SalI | Buffer 3 | 7600 | 6000 | 1600 | |
| ECE165 | EcoRI | HindIII | EcoRI | 5952 | 5200 | 793 |
| ECE166 | EcoRI | HindIII | EcoRI | 7262 | 5100 | 2103 |
| ECE171 | EcoRI | PstI | Fast Digest | 5444 | 3600 | 1826 |
| ECE172 | HindIII | Buffer 2 | 6462 | 3900 | 2540 | |
| ECE176 | EcoRI | XbaI | Fast Digest | 866 | 4250 | 5128 |
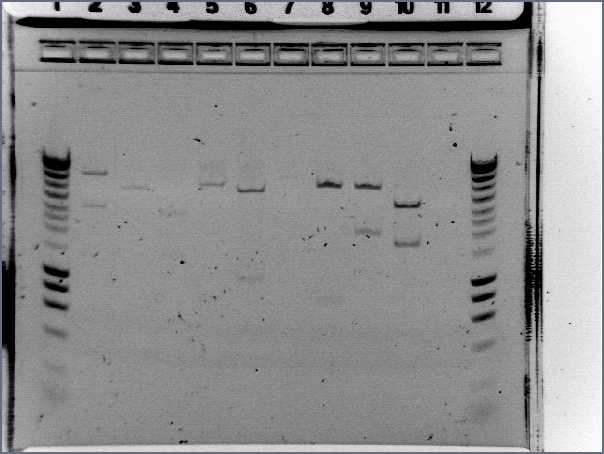
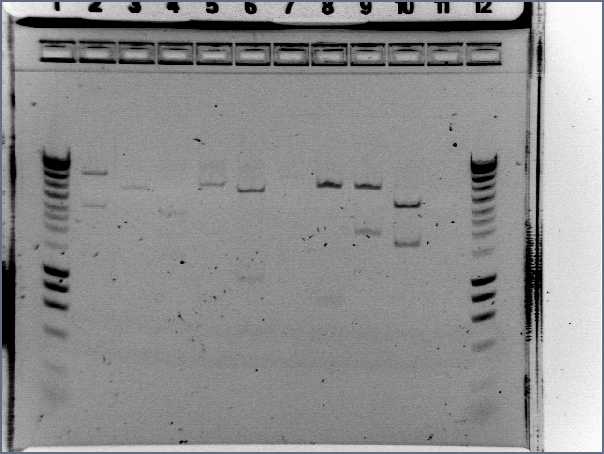
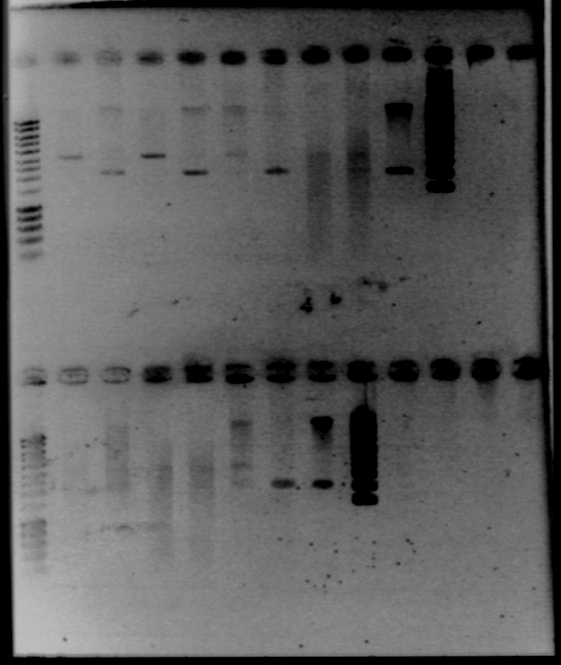
- Results
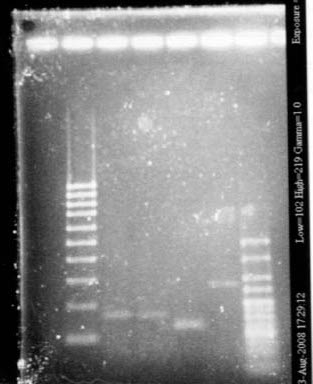
- Lane1 : HyperladderI
- Lane2 : ECE112
- Lane3 : ECE147
- Lane4 : ECE149
- Lane5 : ECE150
- Lane6 : ECE151
- Lane7 : ECE162
- Lane8 : ECE165
- Lane9 : ECE166
- Lane10 : ECE171
- Lane11 : ECE172
- Lane12 : HyperladderI
- ECE112, ECE165, ECE166, ECE171 : right bands, ok
- ECE172 : no band, not enough DNA
- ECE147, ECE150, ECE162 : only one band, maybe not enough DNA
- ECE149 : wrong size!
- ECE151 : not sure, check again
- Plates
- Streak new plates with different strains of Bacillus : 1A1, IA751 and IA771
August 5th
Checking vectors
- Plasmid miniprep for ECE166 (for stock), ECE172 and ECE153
- Digest of ECE153, ECE172 and ECE162 (with plasmid miniprep from yesterday)
- Run on a gel single digest for ECE147 (from yesterday with more DNA), ECE149 (from yesterday with more DNA), ECE150 (from yesterday with more DNA) and ECE 153, ECE162, ECE172
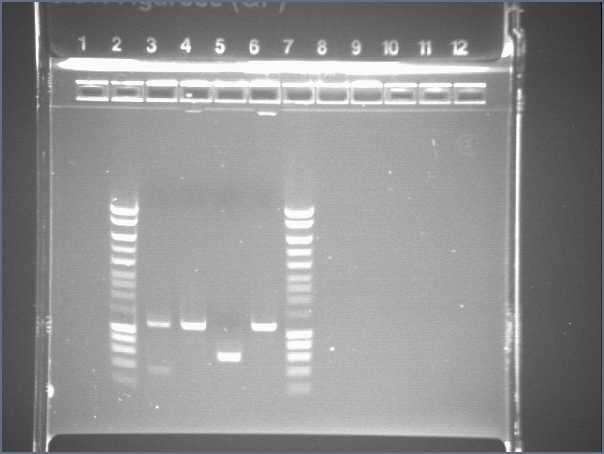
- Results
- Lane3 : HyperladderI
- Lane4 : ECE147
- Lane5 : ECE149
- Lane6 : ECE150
- Lane7 : ECE151
- Lane8 : ECE1162
- Lane9 : ECE172
- Lane10 : hyperladderI
Very low bands : not enough DNA!!!
Transformation of Bacillus
- Result of Nanodrop
| Vector | 260/280 | ng/μL |
|---|---|---|
| ECE112 | 1.75 | 64.6 |
| ECE166 | 172 | 138.6 |
- Prepare medium A with tryptophan, and medium B
- add 5mL of medium A in 3 different tubes, inoculate each tube with colonies (1A1, IA751 ans IA771)
- Check OD every 20min
- Incubate 90min
- Add 0.45mL of medium B and 0.05mL of culture in Ependorf tubes
- Incubate 90min
- Transform : add 1μg of DNA (some with ECE112, some with ECE166) (so you need to nanodrop samples before!)
- Incubate 30min
- Pipette 200μL of solution, spread it on aech plate, wait 10min, and do it again
- Incubate 24hours
- A few glycerol tubes to stock cells : add 2/7 glycerol to cell tubes
- Transformation from glycerol stock from 30/07/2008
- Spin glycerol stocks, pipette out glycerol
- Add 0.5mL of medium B, incubate for 1 hour
- Add 10μL of ECE112 (640ng)
- Incubate 2hours
- Plate 200μL, and 10min later, still 200μL
New stocks
- Do glycerol stock of I746001 and I746101 (no sterile glycerol)
- Put IA751, IA771 in 10mL LB
- Put ECE 176 in 10mL LB + antibiotic
- Reinoculate the tube of LB from yesterday with ECE166 plate
- ECE176 replated onto Amp100 + Cm5
August 6th
Result from transformation
PLATES HAD BEEN BINNED!! OOPS
Check Vectors
- Plasmid miniprep for ECE176 (from 05/08 LB stock and from 04/08 LB stock with a second inoculation)
We want to check uncut plasmid, single and double digest for ECE147, ECE149, ECE150, ECE153, ECE162, ECE172, ECE176. In order to have enough DNA (to see the bands), we will add 1μg of DNA to make single digest, and wr will incubate during 1h (instead of only 10min).
- Nanodrop
| Vetor | 260/280 | μg/mL |
|---|---|---|
| ECE147 | 1.67 | 50.8 |
| ECE149 | 1.66 | 51.9 |
| ECE150 | 1.78 | 107.0 |
| ECE153 | 1.68 | 31.2 |
| ECE162 | 1.56 | 33.1 |
| ECE172 (04/08) | 1.64 | 22.8 |
| ECE172 (05/08) | 1.89 | 213.1 |
| ECE176 (04/08) | 1.89 | 111.1 |
| ECE176 (05/08) | 1.83 | 96.2 |
We made singe digest for the samples from 05/08 only.
- Double digest (1 hour of incubation in water bath at 37°C)
- Gel
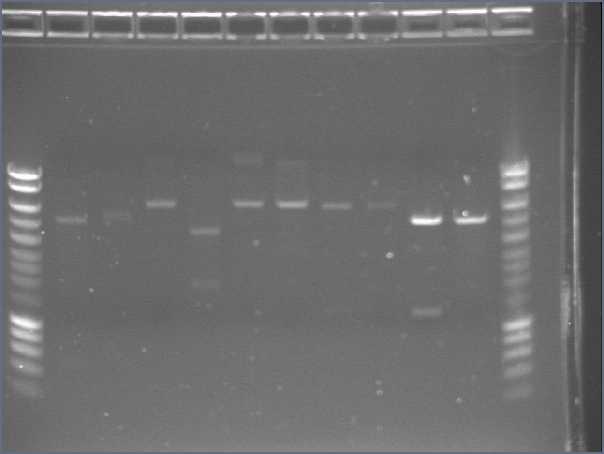
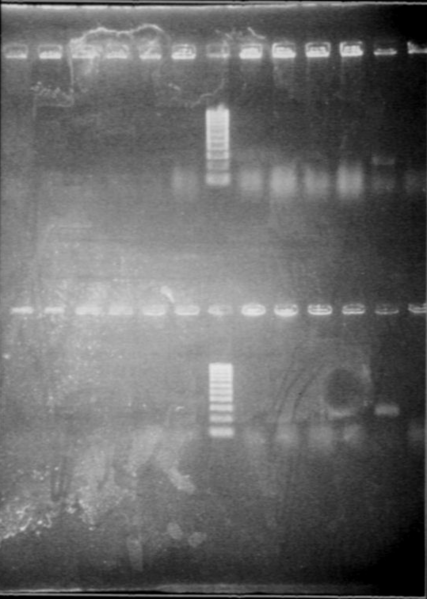
Gel1
- Lane1 : HyperladderI
- Lane2 : ECE147 with EcoRI
- Lane3 : ECE147 with HindIII
- Lane4 : ECE149 with SpeI
- Lane5 : ECE149 with PstI
- Lane6 : ECE150 with SpeI
- Lane7 : ECE150 with HindIII
- Lane8 : ECE153 with SalI
- Lane9 : ECE162 with SalI
- Lane10 : ECE172 with HindIII
- Lane11 : ECE176 with EcoRI
- Lane12 : HyperladderI
Gel2
- Lane1 : HyperladderI
- Lane2 : ECE176 with XbaI
- Lane3 : ECE176 double digest
- Lane4 : ECE150 double digest
- Lane5 : ECE149 double digest
- Lane6 : ECE147 double digest
- Lane7 : HyperladderI
- Lane8 : ECE149 uncut
- Lane9 : ECE153 uncut
- Lane10 : ECE172 uncut
- Lane11 : supercoiled ladder
- Results
Our supercoiled ladder was very bad, so it was impossible to conclude for uncut vectors.
- ECE147, ECE 150, ECE153, ECE 176 single digest : ok
- ECE149 : problem, 2 cutting sites for PstI (only one in the sequence)
- ECE162 : only one band whereas SalI should have 2 cutting sites
- ECE172 : wrong sizes!!!
- Double digest for ECE176 : ok!
- Double digest for ECE 150, 149 and 147 : only one band!!!
There may be a problem when we do the double digest by adding two single digest. We will try again with a direct double digest.
August 7th
Transformation of B.S. IA771
New transformation with the same protocol than 2 days ago. We will try to add less liquid on plates (to avoid growing colonies in liquid)
| Tube | Plate 1 | Plate 2 | Plate 3 | Plate 4 |
|---|---|---|---|---|
| ECE166 | Cm5 + 200μL of cells | Cm5 + 100μL of cells | Cm5 + 50μL of cells | Cm10 + 100μL of cells |
| ECE153 | Spc50 + 200μL of cells | Spc50 + 100μL of cells | Spc50 + 50μL of cells | |
| ECE166 | Cm5 + 100μL of cells | Cm10 + 100μL of cells | ||
| ECE153 | Spc50 + 100μL of cells | |||
| no DNA | Cm5 + 100μL of cells | Cm10 + 100μL of cells | Spc50 + 100μL of cells | |
| ECE166 (glycerol stock) | Cm5 + 100μL of cells | Cm10 + 100μL of cells |
Check Vectors
We want to make a final check for ECE147, 149, 150, 153 and 162.
- Double digest + gel
- Results
- Lane3 : HyperladderI
- Lane4 : ECE147
- Lane5 : ECE149
- Lane6 : ECE150
- Lane7 : ECE153
- Lane8 : ECE162
- Lane9 : HyperladderI
- ECE 147, ECE150, ECE153 :ok!
- ECE 149 : 3 bands! not the right vector
- ECE162 : ony one band! It has been uncut... maybe the good vector (according to precedent gel), but not sure
Test ECE112 transformation
- for the three different strain (1A1, IA771, IA751), make 12 spots
- take one colony with a loop, and put it on a Cm5 plate and on a Cm5 + Spc100 plate (do that for the 12 spots)
- incubate
New stock
- Put ECE171 in 10mL of LB
- Incubate
August 8th
Result from yesterday transformation
| Vector | Antibiotic | Quantity of cells added | number of colonies |
|---|---|---|---|
| ECE166 | Cm5 | 200μL | 0 : problem!!! |
| ECE166 | Cm5 | 100μL | 2 |
| ECE166 | Cm5 | 50μL | 1 |
| ECE166 | Cm10 | 100μL | 0 : no resistance to Cm10! |
| ECE166 (spin) | Cm5 | 100μL | a lot, confluent |
| ECE166 (spin) | Cm10 | 100μL | 0 |
| ECE166 (glycerol + spin) | Cm5 | 100μL | about 150, very small |
| ECE166 (spin) | Cm10 | 100μL | 0 |
| ECE153 | Spc50 | 200μL | 21 + a lot of confluent colonies |
| ECE153 | Spc50 | 100μL | 7 + confluent (a few) |
| ECE153 | Spc50 | 50μL | 4 |
| ECE153 (spin) | Spc50 | 100μL | 25 + about 200 (small and almost confluent) |
| No DNA | Cm5 | 100μL | 0 |
| No DNA | Cm10 | 100μL | 0 |
| No DNA | Spc50 | 100μL | about 12, maybe more : problem!!!!! |
Result for test of ECE112 transformation
On Cm5 plates, we have some colonies for each strains, on Cm5 + Spc 100 plates, no colonies on each plates. This result seems good, however, we forgot to make a control. Since, we had some colonies on control plates, it is possible that our bacillus are resistant to Cm5, even if they are not transformed. We addded some control colonies.
Control with erythromycin
- Prepare erythromycin, stock 5mg/mL : 0.05g in 10mL of SDW
- Prepare plates : add 20μL of Ery (5mg/mL) into 200mL of agar (final concentration : 0.5μg/mL)
- Do several spots on each plate
| Plate | Colonies added from |
|---|---|
| 1 | ECE166 + 100μL of cells (2 different plates) |
| 2 | ECE153 + 100μL of cells (2 different plates) |
| 3 | ECE166 (from glycerol stock) |
| 4 | Control (from a plate of IA771 without antibiotic) |
| 5 | IA771 + ECE112 (05/08) |
| 6 | IA771 + ECE166 (05/08) |
Amylase production screening
- Prepare agar plate with starch
- Starch : 1g of starch in 10mL of SDW
- Add 2mL of Starch solution into 200mL of agar, mix
-Inoculate on different plates : 1A1 +ECE112 (plate from 05/08), IA751 + ECE112 (plate from 05/08) and IA771 + ECE153
New stocks
- Take 1mL from LB stock oh ECE171 (from 07/08) and put it into 99mL of LB
August 11th
Erytromycin Experiment
- New preparation of Ery : 0.05g into 10mL of ETHANOL
- We plated again our plates (same than 08/08/08)
Amylase screening experiment
- New method : add 2g of starch powder into 200mL of Agar, shake, pipette to plate (and avoid bubbles)
- Same plates than 08/08/08
Test our stock of ECE171
-Plasmid miniprep 9from the samples of 10mL and the one of 100mL)
-Nanodrop both DNA
| Vetor | 260/280 | μg/mL |
|---|---|---|
| ECE171 (10mL) | 1.73 | 136.3 |
| ECE171(100mL) | 1.82 | 99.5 |
- Double digest (EcoRI and PstI) with 10μL of DNA and 1h of incubation
- Samples have been frozen, they should be run onto a gel
Control of Spc resistance of Bacillus
- Spc50 plate with transformed IA771 (ECE153, which is Spc resistant) and with IA771 9which should not be Spc resistant)
August 12th
Results from yesterday
- Ery plates
- IA751 (control) : most of colonies did not grow, which is good, but 2 or 3 grew...
- IA7771 + ECE166 : all colonies grew : ok 9non integration vector)
- IA771 + ECE153 : growth of all colonies... problem!!! if IA771 is transformed with this integration vector, it should lose its EryR
- IA771 + ECE112 : growth of all colonies... problem!!! if IA771 is transformed with this integration vector, it should lose its EryR
- Spc resistance
- IA771 : no growth on Spc50 plate, so no natural resistance
- transformed cells grew
- Amylase plates
- Add iodine solution for 1min
- Problem : no aparent blue, no diffusion of iodine into LA agar + a lot of contamination
New plates and LB stocks
- In order to try to make plasmid miniprep at the end of the day, grow IA771 transformed with ECE166 in 10mL LB + Cm5
7hours is not enough to reach exponential phase!!! We will try again tomorrow by incubating longer
- Grow IA771 and IA751 ( first plate from sterile disks of strain) imto 10mL of LB
- Grow ECE166, 171, 153 in E.coli with approriate antibiotics 9to transform tomorrow)
- Grow IA771 transformed with ECE153 into LB+Spc50 (to check fluorescence with xylose induction)
New test for amylase
We tried 2 different methods.
- Dilute 1g of starch into 100mL of agar and try to dilute it and boil it to sterilize. The problem is that it is very difficult to dilute...
- The second method seem to be better. We diluted 1g of starch into 100mL of Soft Agar (it dilutes very well). Then plate blank agar plate (LA) and then add a thin layer of SA ith 1% of starch. Poke the plates.
- We plated IAI+ECE112, IA751+ECE112 and also IA751 and 1A1 for control.
August 16th
Agr A and C ligation to pSB4C5
Ligation was performed using Fermentas Rapid Ligation kit according to their protocol.
After ligation, samples were visualized on a gel, but little product of the correct size was seen.
For agrA we should have gotten a band of size 3700 (3000plasmid + 700 insert)
For agrC we should have gotten a band of size 4300 (3000plasmid + 1300insert)
The bands of appropriate sizes were extracted for transformation.
August 18th
Check promoters
On Friday, PCR out promoters, we want to check them.
- Run a gel
- Results : good size of bands!!!
Transformation of AgrA and AgrC
AgrA and C gell-extracted ligation was transformed into Top 10 cells using standard protocol. Puc9 was used as a positive control.
August 19th
Results of AgrA and C transformation
Transformation has failed. No colonies were visible for the plates of Agr A or C. The Puc9 positive control grew. We believe that the problem was in the gel-extraction between ligation and transformation. Most of our plasmid was probably lost in this step. Next time we will directly use the results from the ligation reaction to transform. Although many bands were seen on the previous gel of the ligation reaction, we will check our transformation growth by single colony PCR to confirm transformation of the plasmid with our correct insert.
Lux parts
To make the Lux Receiver, we need 4 different parts ;
- R0040, TetR repressible promoter
- SO168, luxR + double terminator
- R0062, promoter activated by luxR
- JO4630 (GFP + double terminator)
All these parts have been transformed into E.coli. We want to test them. R004, R0062 and JO4630 have already been tested, it should be fine. We received from the MIT R0040, R0062 and S068 already transformed into E.coli, so we want to check these stocks (which are certainly fine) and use them. For JO4630, we want to double check our transformation.
- Plate on antibiotic plates and do LB stocks of single colony from the MIT stock (R0040, R0062 and S0168).
- Put on Kan plates 4 different colonies from J04630 (transformation Amp plate) and also incubate these colonies into LB
August 20th
Check Lux components
- Single colony PCR for :
- R0040 (MIT stuff)
- R0062 (MIT stuff)
- 4 different colonies of S0168 (from a transformation plate from 12/08)
- 4 different colonies of J04630 (from a transformation plate from 12/08)
- Protocol : add 1μL of cells (diluted in water), 10μL of Master Mis, 7μL of SDW, 1μL of VF primer and 1μL of VR primer
- Gel PCR products
Gel 1
- Lane2 : Hyperladder1
- Lane3 : JO4630, colony 1
- Lane4 : JO4630, colony 2
- Lane5 : JO4630, colony 3
- Lane6 : JO4630, colony 4
- Lane7 : HyperladderI
Gel 2
- Lane2 : HyperladderI
- Lane3 : ECE190 double digest
- Lane4 : S0168, colony 1
- Lane5 : S0168, colony 2
- Lane6 : S0168, colony 3
- Lane7 : S0168, colony 4
- Lane8 : HyperladderI
- Lane9 : R0040
- Lane10 : R0062
- Lane11 : Ladder 100bp
- Results
- R0040 and R0062 : one big band of about 300b (expected size 293), OK!
- S0168 : one band of about 400b for the 4 different colonies (expected size 1234!), bad! This plate does not contain S0168
- J04630 (colonies 2 and 4) : one band of about 1100b (expected size 1173), OK!
- J04630 (colony 1) : one good band plus another band...
- J04630 (colony 3) : one band of about 600b, bad!
Ligation
- Materials :
- AgrA
- AgrB
- AgrC
- AgrD
- Pupp
- Pspac
- Ppac
- Pxyl
- RBS S
- RBS W
- psB4C5
- Double digest of PCR products
- Run vector, AgrA and AgrD on a gel
- DNA clean and concentrator for AgrA, B,C and D, promoters
- Microclean for both RBS
- Nanodrop
| 260/280 | ng/μL | |
|---|---|---|
| AgrA | 1.66 | 16.4 |
| AgrB | 1.91 | 23.5 |
| AgrC | 1.99 | 35.9 |
| AgrD | 2.13 | 4.9 |
| Pxyl | 1.54 | 5.6 |
| Ppac | 1.49 | 4.6 |
| Pspc | 1.62 | 9.6 |
| Pupp | 1.88 | 8.5 |
| RBS S | 2.44 | 29 |
| RBS W | 1.44 | 10.7 |
- Extract plasmid annd Agr from gel and clean
- Ligation
August 21st
Transformation of ligation products
- Spin chemically competent TOP10, add 100μL of CaCl2 solution
- Add 5μL of DNA (ligation products), and 1μL of PUC9
- Continue the protocol of transformation
J04630
- Plate good colonies from yesterday on Kan25 and put in LB (for plasmid stock for tomorrow)
Plasmid stocks
- Plasmid miniprep R0040 and R0062
August 23rd
Check promoters (after PCR from bacillus vectors)
- Gel
- Lane1 : Hyperladder 5
- Lane2 : Pxyl
- Lane3 : Pspac
- lane4 : Ppac
- Lane5 : Pupp
Size is ok.
August 27th
New transformation of ligation products
- Products : agrD, Pupp, Pspac, RBS W, RBS S (from ligation with biolabs kit)
- Competent top 10 from the freezer
- Add 5μL of ligation products, and 1.2μL of PUC9
August 28th
Results from transformation of ligation products
- nothing on plate, even on control plate
- Reasons ?
- cells non competent anymore (try with fresh competent cells)
- not enough DNA (try with 10μL of DNA)
- very short ligation products
Transformation of ligation products (new)
- Products to ligate : Pupp, Pspac, agrD, RBS S and RBS W
- new fresh competent TOP 10
- 5μL of DNA (1.5μL of PUC9)
- 2h30 in the incubator
August 29th
Results of transformation with our ligation products
- Everything grew! Better efficiency with electrop. than with chemical protocol
Single colony PCR to check our transformation
| Transformed products | number of picked colonies from chemical transformation | number of picked colonies from electrop. transformation (neat) | number of picked colonies from electrop. transformation (1:10) |
|---|---|---|---|
| Pupp | 2 | 2 | 0 |
| Pspac | 2 | 2 | 0 |
| RBS S | 3 | 0 | 2 |
| RBS W | 3 | 0 | 2 |
| agrD | 3 | 2 | 0 |
- Single colony PCR : 13μL of SDW+cells, 5μL of Master Mix, 1μL of VF and 1μL of VR (and plate each single colony)
- Load a gel (1.3% agarose) : 5μL of PCR products + 1μL of dye (only 1μL of 100b ladder)
In the death plasmid, the VF-VR size is about 280b.
| Transformed products | size of the product (with cutting sites) | expected size after PCR (about) |
|---|---|---|
| Pupp | 255 | 480 |
| Pspac | 125 | 350 |
| RBS S | 56 | 280 |
| RBS W | 56 | 280 |
| agrD | 200 | 430 |
- Result : nothing, even no ladder, problem with the gel!
- run again on a e-gel
- Lane1 :ladder 100bp
- Lane2 : Pupp colony 1
- Lane3 : Pupp colony 3
- Lane4 : Pspac colony 1
- Lane5 : Pspac colony 3
- Lane6 : RBS S colony 1
- Lane7 : RBS S colony 4
- Lane8 : RBS W colony 1
- Lane9 : RBS W colony 4
- Lane10 : agrD colony 1
- Lane11 : agrD colony 4
- Lane12 : HyperladderI
- Result : nothing, just the primers! Problem with our PCR
Plate biobricks from MIT
- E0840
- B0014
- I712007
- C0012
- B0015
- C0061
- R0063
September 2nd
Check transformation in E.coli of our ligated products
- Single colony PCR with VF and VR : add 10μL of SDW, 5μL of MM, 1μL of VF, 1μL of VR and 3μL of cells
| inserted products | number of colonies to check (chemical transf.) | number of colonies to check (electrop. transf.) |
|---|---|---|
| RBS S | 3 | 2 |
| RBS W | 3 | 2 |
| Pspac | 2 | 2 |
| Pupp | 2 | 2 |
| agrD | 3 | 2 |
- Gel (3% of agarose)
- Result
| gel | lane | inserted product (name of the colony) | observation | conclusion |
|---|---|---|---|---|
| top | 3 | RBS S (1) | one band (260bp) | size of the insert, RBS too small to see on a gel |
| top | 4 | RBS S (2) | one band (260bp) | size of the insert, RBS too small to see on a gel |
| top | 5 | RBS S (4) | nothing | ? |
| top | 6 | RBS S (5) | nothing | ? |
| top | 7 | RBS W (1) | 2 band (260bp + 350bp) | size of the insert, RBS too small to see on a gel + something else? |
| top | 8 | RBS W (2) | one band (350bp) | too big |
| top | 9 | RBS W (4) | one band (260bp) | size of the insert, RBS too small to see on a gel |
| top | 10 | RBS W (5) | one band (260bp) | size of the insert, RBS too small to see on a gel |
| top | 11 | Pspac (1) | 2 band (260bp + 350bp) | maybe problem with products loaded on gel (exactly the same bands than for RBS W) |
| top | 12 | Pspac (2) | one band (450bp) | size of Pupp? |
| top | 13 | Pspac (3) | one band (450bp) | size of Pupp? |
| bottom | 3 | Pspac (4) | nothing | ? |
| bottom | 4 | Pupp (1) | one band (400bp) | size of Pspac? |
| bottom | 5 | Pupp (2) | one band (400bp) | size of Pspac? |
| bottom | 6 | Pupp (3) | one band (slightly lower than 400bp) | size of Pspac? |
| bottom | 7 | Pupp (4) | one band (400bp) | size of Pspac? |
| bottom | 8 | agrD (1) | 2 bands (380 and 450bp) | big band is ok |
| bottom | 9 | agrD (2) | 2 bands (380 and 450bp) | big band is ok |
| bottom | 10 | agrD (3) | no bands | ? |
| bottom | 11 | agrD (4) | strong band (450bp) | ok |
| bottom | 12 | P2 | one band (350bp) | ok |
September 3rd
Checking the insert of promoters
We ligated promoters into death vector, and transforned into Top 10. Our transformation worked, but it was hard to say with the result of yesterday if we had the good insert. Moreover, it is possible that we inverted Pupp and Pspac. So we are going to make a new single colony PCR with the primers of promoters (we used the same single colonies than yesterday).
We also want to PCR again all promoters (Pspac, Pxyl, Pupp and Ppac) from plasmids to have more.
Picture of the gel with the different promoters
| promoter | colony | primers | name |
|---|---|---|---|
| Pspac | 1 | Pspac primers | A |
| Pspac | 1 | Pupp primers | B |
| Pspac | 2 | Pspac primers | C |
| Pspac | 2 | Pupp primers | D |
| Pupp | 1 | Pupp primers | E |
| Pupp | 1 | Pspac primers | F |
| Pupp | 3 | Pupp primers | G |
| Pupp | 3 | Pspac primers | H |
- Single colony PCR : add 10μL of SDW, 5μL of MM, 1μL + 1μL of primers, 3μL of cells
- Gel
- lane 3 : hyperladderI
- lane 4 : Pspac (colony1) with Pspac primers
- lane 5 : Pspac (colony1) with Pupp primers
- lane 6 : Pspac (colony2) with Pspac primers
- lane 7 : Pspac (colony2) with Pupp primers
- lane 8 : Pupp (colony1) with Pupp primers
- lane 9 : Pupp (colony1) with Pspac primers
- lane 10 : Pupp (colony3) with Pupp primers
- lane 11 : Pupp (colony3) with Pspac primers
- lane 12 : HyperladderI
- Results
| promoter | colony | primers | observation | Conclusion |
|---|---|---|---|---|
| Pspac | 1 | Pspac primers | nothing | it is in fact Pupp |
| Pspac | 1 | Pupp primers | band (about 280bp) | Pupp OK |
| Pspac | 2 | Pspac primers | nothing | it is in fact Pupp |
| Pspac | 2 | Pupp primers | band (about 280bp) | Pupp OK |
| Pupp | 1 | Pupp primers | nothing | it is in fact Pspac |
| Pupp | 1 | Pspac primers | band (about 180bp) | Pspac OK |
| Pupp | 3 | Pupp primers | nothing | it is in fact Pspac |
| Pupp | 3 | Pspac primers | band (about 180bp) | Pspac OK |
Make some stock of our new biobricks
- Pupp (colony1) into the death vector, transformed into TOP10
- Pspac (colony1) into the death vector, transformed into TOP10
- agrD (colony5) into the death vector, transformed into TOP10
- Grow this single colony into 10mL of LB (Cm35)
Transformation of new "biobricks"
- transform into E.coli :
- agrA
- agrB
- agrC
- Pxyl
September 4th
Results of new "biobricks"transformation
- Nothing on our plate, except for the control plate.
New attempt of new "biobricks" transformation
- transform into E.coli these ligated products:
- agrA
- agrB
- agrC
- Pxyl
September 7th
New PCR
- New stocks of Master Mix
- PCR agrA, agrB, Pxyl, Pspac, Ppac and Pupp
September 8th
Check product of PCR from yesterday
- Lane3 : Hyperladder I
- Lane4 : agrA
- Lane5 : agrB
- Lane6 : Pxyl
- Lane7 : Pspac
- Lane8 : Ppac
- Lane9 : Pupp
- Lane10 Hyperladder IV
- Results : everything is ok
Check transformation from 03/09
After several days of incubation, we have a few colonies on our transformed plates (agrA, B and C).
- Single colony PCR
- Add 13μL of cells diluted into SDW, 5μL of MM, 1μL of VF and 1μL of VR
- Gel
September 10th
PCR of GFP+RBS and Promoter+RBS
- PCR
- Run a 1.2% agarose gel with 1μL of sample
- Lane 3 : HyperladderI
- Lane 4 : GFP + RBS 1A
- Lane 5 : GFP + RBS1B
- Lane 6 : GFP + RBS 2A
- Lane 7 : GFP + RBS 2B
- Lane 8 : Pupp + RBS 1
- Lane 9 : Pupp + RBS 2
- Lane 10: HyperladderIV
- Result
RBS screening
- 5 colonies from chemical transformation + 6 colonies from electroporation transformation for RBS S
- 5 colonies from chemical transformation + 6 colonies from electroporation transformation for RBS W
- PCR : 11μL of SDW + 5μL of MM + 1+1μL of primers (RBS detect + VR) + 2μL of cells (program iGEM34)
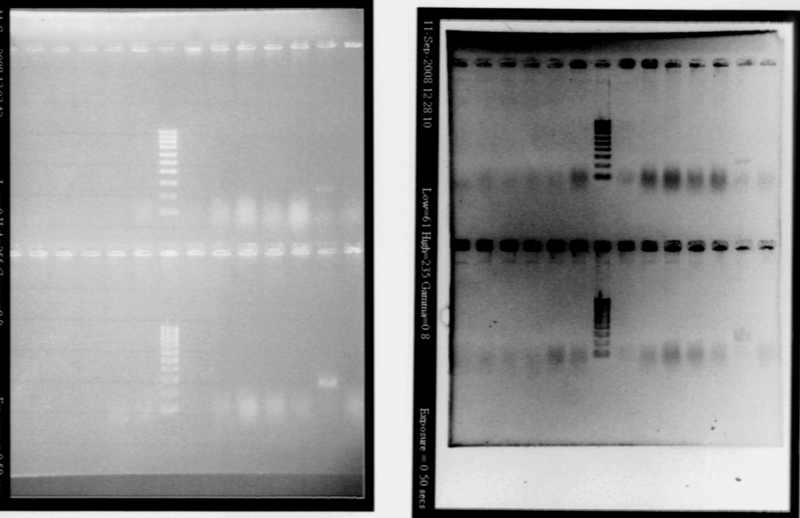
- Gel1
- Lane2 : RBSS1
- Lane3 : RBS S2
- Lane4 : RBS S3
- Lane5 : RBS S4
- Lane6 : RBS S5
- Lane7 : RBS S6
- Lane8 : HyperladderIV
- Lane9 : RBS S7
- Lane10 : RBS S8
- Lane11 : RBS S9
- Lane12 : RBS S10
- Lane13 : RBS S6 with VF and VR primers
- Lane14 : RBS S11
- Gel2
- Lane2 : RBSW1
- Lane3 : RBS W2
- Lane4 : RBS W3
- Lane5 : RBS W4
- Lane6 : RBS W5
- Lane7 : RBS W6
- Lane8 : HyperladderIV
- Lane9 : RBS W7
- Lane10 : RBS W8
- Lane11 : RBS W9
- Lane12 : RBS W10
- Lane13 : RBS W6 with VF and VR primers
- Lane14 : RBS W11
- Result
- Nothing, except for the PCR with VF and VR primers, either our primers are not right, either we have no insert. But the gel is not good enough to see very well, we will try to run a new gel.
September 11th
RBS screening
- We run our PCR products from yesterday on a new 1.8% agarose gel
- Gel1
- Lane2 : RBSS1
- Lane3 : RBS S2
- Lane4 : RBS S3
- Lane5 : RBS S4
- Lane6 : RBS S5
- Lane7 : RBS S6
- Lane8 : HyperladderIV
- Lane9 : RBS S7
- Lane10 : RBS S8
- Lane11 : RBS S9
- Lane12 : RBS S10
- Lane13 : RBS S6 with VF and VR primers
- Lane14 : RBS S11
- Gel2
- Lane2 : RBSW1
- Lane3 : RBS W2
- Lane4 : RBS W3
- Lane5 : RBS W4
- Lane6 : RBS W5
- Lane7 : RBS W6
- Lane8 : HyperladderIV
- Lane9 : RBS W7
- Lane10 : RBS W8
- Lane11 : RBS W9
- Lane12 : RBS W10
- Lane13 : RBS W6 with VF and VR primers
- Lane14 : RBS W11
- Result
- Nothing, except for the PCR with VF and VR primers, either our primers are not right, either we have no insert.
RBS screning but single digest
- Grow some colonies of Top10 transformed with RBS into 10mL of LB with antibiotic
We want to check our transformation with single digest. If we have self ligation in our transformation, we will loose the XbaI cutting site.
Backbone for ligation
- New pellets in 600μL of SDW
- Plasmid miniprep
September 12th
RBS screening (single digest)
- Plasmid miniprep 6 colonies of RBS S and 6 colonies of RBS W (without endo wash buffer)
- Nanodrop
| product | concentration (ng/μL) | 260/280 | quantity of DNA to add to have about 300ng (μL) | added SDW for single digest (μL) |
|---|---|---|---|---|
| RBS W1 | 57.1 | 1.57 | 6 | 11 |
| RBS W3 | 54.1 | 1.53 | 6 | 11 |
| RBS W5 | 15.1 | 1.85 | 17 | 0 |
| RBS W 7 | 21.8 | 1.66 | 15 | 2 |
| RBS W9 | 21.8 | 1.78 | 15 | 2 |
| RBS W11 | 13.8 | 1.73 | 17 | 0 |
| RBS S2 | 19.5 | 1.7 | 15 | 2 |
| RBS S4 | 82.13 | 2.57 | 4 | 13 |
| RBS S6 | 44.7 | 1.64 | 7 | 10 |
| RBS S8 | 104.0 | 1.48 | 3 | 14 |
| RBS S10 | 13.1 | 1.82 | 17 | 0 |
| RBS S11 | 20.1 | 1.73 | 15 | 2 |
- Single digest : with EcoRI and XbaI, add SDW and DNA (according to the previous table, 300mg of DNA), 2μL of fast digest buffer, and 1μL of enzyme
- Gel1
- Lane2 : HyperladderI
- Lane3 : RBS W1 cut with EcoRI
- Lane4 : RBS W1 cut with XbaI
- Lane5 : RBS W3 cut with EcoRI
- Lane6 : RBS W3 cut with XbaI
- Lane7 : RBS W7 cut with EcoRI
- Lane8 : RBS W7 cut with XbaI
- Lane9 : RBS W9 cut with EcoRI
- Lane10 : RBS W9 cut with XbaI
- Lane11 : RBS W9 uncut
- Lane12 : supercoiled ladder
- Gel2
- Lane2 : HyperladderI
- Lane3 : RBS S4 cut with EcoRI
- Lane4 : RBS S4 cut with XbaI
- Lane5 : RBS S6 cut with EcoRI
- Lane6 : RBS S6 cut with XbaI
- Lane7 : RBS S8 cut with EcoRI
- Lane8 : RBS S8 cut with XbaI
- Lane9 : RBS S11 cut with EcoRI
- Lane10 : RBS S11 cut with XbaI
- Lane11 : RBS S4 uncut
- Lane12 : supercoiled ladder
- Result
The size of our plasmid is about 3000bp (ok on the gel)
- RBS W
- W1 with EcoRI : cut
- W1 with XbaI : uncut, self ligation
- W3 with EcoRI : cut
- W3 with XbaI : uncut, self ligation
- W7 with EcoRI : impossible to see
- W7 with XbaI : uncut, self ligation
- W9 with EcoRI : cut
- W9 with XbaI : uncut, self ligation
- RBS S
- S4 with EcoRI : cut
- S4 with XbaI : uncut, self ligation
- S6 with EcoRI : cut
- S6 with XbaI : 2 band, this plasmid is partially cut!!! Transformation with RBS S
- S8 with EcoRI : cut
- S8 with XbaI : uncut, self ligation
- S11 with EcoRI : cut
- S11 with XbaI : uncut, self ligation
Double check of RBS S6 and stock
- Grow RBS S6 in LB with Cm35
Check more RBS W
- Grow RBS W2, 4, 6, 8, 10 in 10mL of LB with antibiotic
Check PCR products
- Gel (2μL of each product)
- Lane1 : λ ladder
- Lane2 : agrA
- Lane3 : agrB
- Lane4 : agrC
- Lane5 : rep
- Lane6 : pSB4C5 1
- Lane7 : pSB4C5 2
- Lane8 : Pxyl
- Lane9 : Ppac
- Lane10 : RBS S
- Lane11 : RBS W
- Lane12 : HyperladderI
- Results
- agr B : 2 bands?
- agrC : ok
- rep : small band but good size
- backbones : ok
- promoters : ok
- RBS : nothing
September 13th
Grow RBS S6
- Put the 10mL of LB with RBS S6 from yesterday into 200mL of LB with antibiotic
- Incubate at 37°C with shaking
RBS W screening
- Plasmid miniprep of RBS W2, 4, 6, 8, 10 9witouh endo wash buffer)
- Nanodrop
| product | concentration (ng/μL) | 260/280 | quantity of DNA to add to have about 300ng (μL) | added SDW for single digest (μL) |
|---|---|---|---|---|
| RBS W2 | 42.4 | 1.62 | 8 | 9 |
| RBS W4 | 35.1 | 1.61 | 10 | 7 |
| RBS W6 | 41.0 | 1.67 | 8 | 9 |
| RBS W8 | 72.8 | 1.61 | 5 | 12 |
| RBS W10 | 51.0 | 1.60 | 6 | 11 |
- Single digest with EcoRI and XbaI
- Gel1
- Lane2 : HyperladderI
- Lane3 : RBS W2 cut with EcoRI
- Lane4 : RBS W2 cut with XbaI
- Lane5 : RBS W4 cut with EcoRI
- Lane6 : RBS W4 cut with XbaI
- Lane7 : RBS W5 cut with EcoRI
- Lane8 : RBS W5 cut with XbaI
- Lane9 : RBS W6 cut with EcoRI
- Lane10 : RBS W6 cut with XbaI
- Lane11 : RBS W8 uncut
- Lane12 : supercoiled ladder
- Gel2
- Lane2 : HyperladderI
- Lane3 : RBS W8 cut with EcoRI
- Lane4 : RBS W8 cut with XbaI
- Lane5 : RBS W10 cut with EcoRI
- Lane6 : RBS W10 cut with XbaI
- Lane7 : RBS W11 cut with EcoRI
- Lane8 : RBS W11 cut with XbaI
- Lane9 : RBS W8 uncut
- Lane10 : supercoiled ladder
- Result
- W2 with EcoRI : cut
- W2 with XbaI : uncut, self ligation
- W4 with EcoRI : cut
- W4 with XbaI : uncut, self ligation
- W5 with EcoRI : cut
- W5 with XbaI : uncut, self ligation
- W6 with EcoRI : cut
- W6 with XbaI : cut (2 bands), transformation ok for RBS W6
- W8 with EcoRI : impossible to see
- W8 with XbaI : impossible to see
- W10 with EcoRI : cut
- W10 with XbaI : maybe cut, it seems to be 2 bands, but quite difficult to see
- W11 with EcoRI : cut
- W11 with XbaI : uncut, self ligation
Double check of RBS W6 and stock
- Grow RBS W6 in 10mL of LB with antibiotic
 "
"