Team:Chiba/Calendar-Home/16 October 2008
From 2008.igem.org
15 October 2008 <|> 17 October 2008
Laboratory work
Team:Demo-Is
- Pre-culture
- Picked and cultured the following glycerol stocks in 2mL of LB:
- LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002], (JW1908)
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), (XL10G)
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)]), (XL10G)
- Cultured at 37°C for 12h.
- Picked and cultured the following glycerol stocks in 2mL of LB:
- Culture
- Added 6.25% each of the pre-cultures to new LB medium.
- LB-Amp, [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]
- LB-Amp+0.2%Glu, [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0161 LuxI(no LVA)]), [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]([http://partsregistry.org/Part:BBa_J04500 plac+rbs]+[http://partsregistry.org/wiki/index.php?title=Part:BBa_C0178 LasI(no LVA)])
- Cultured at 37°C for 4~5h
- Added 6.25% each of the pre-cultures to new LB medium.
- Wash
- Transfer 10mL each of the culture to 50mL centrifuge tubes.
- Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant.
- Added LB-Amp to each centrifuge tube:
- 10mL to the tube that contains [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]
- 5mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Centrifuged for 6min, 3600rpm at 20°C the tube containing [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant.
- 10mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Centrifuged for 6min, 3600rpm at 20°C the tube containing [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] and discarded the supernatant.
- 5mL to the tube that contains [http://partsregistry.org/Part:BBa_K084012 BBa_K084012], [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]
- Mix
- Mixed the sender cells [http://partsregistry.org/Part:BBa_K084012 BBa_K084012] and [http://partsregistry.org/Part:BBa_K084007 BBa_K084007] both with [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] at a 1:1 ratio.
- Added 100μL each to a 96-well shallow plate (as shown in the figure).
- Green part is [http://partsregistry.org/Part:BBa_K084012 BBa_K084012]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1
- Red part is [http://partsregistry.org/Part:BBa_K084007 BBa_K084007]:[http://partsregistry.org/Part:BBa_T9002 BBa_T9002]=1:1
- Uncolored part is [http://partsregistry.org/Part:BBa_T9002 BBa_T9002] alone.
- Culture and observe results
Results
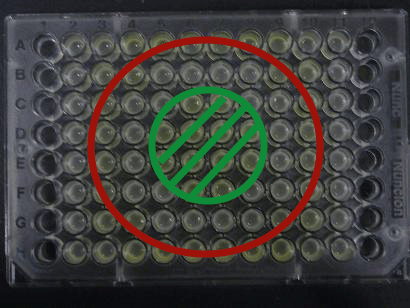 Green region: sender=LuxI,
Red circular region: sender=Las I.
Green region: sender=LuxI,
Red circular region: sender=Las I.
LuxI GFP is detected at 4h following mixing while LasI GFP is detected
after 8h, thus successfully demonstrating time-delay depending on the
sender used.
 "
"