Team:Chiba/Calendar-Home/21 August 2008
From 2008.igem.org
(→Team:Input) |
(→Team:Input) |
||
| Line 19: | Line 19: | ||
| - | *UV irradiation test (plate | + | *UV irradiation test (plate phase) |
*two plasmids from --- | *two plasmids from --- | ||
**(Repressor)-Ptrc-LuxR-Plux-cI-colE1-Amp- | **(Repressor)-Ptrc-LuxR-Plux-cI-colE1-Amp- | ||
Revision as of 01:08, 30 October 2008
20 August 2008 <|> 22 August 2008
Contents |
Laboratory work
Team:Input
(-->20/8)
Inoculated transformants for 12 hours in LB 2mL containing Ampicillin.
- [http://partsregistry.org/Part:BBa_R0051 BBa_R0051](2007)
- [http://partsregistry.org/Part:BBa_R0051 BBa_R0051](2006)
- [http://partsregistry.org/Part:BBa_J06650 BBa_J06650](2007)
- [http://partsregistry.org/Part:BBa_J06650 BBa_J06650](2006)
- [http://partsregistry.org/Part:BBa_J22136 BBa_J22136](2007)
- [http://partsregistry.org/Part:BBa_J22141 BBa_J22141](2007)
BBa_J22141:After inoculated for 11 hours, added 20μl IPTG and cultured one hour.
- UV irradiation test (plate phase)
- two plasmids from ---
- (Repressor)-Ptrc-LuxR-Plux-cI-colE1-Amp-
- (Reporter)-PcI-GFP-p15a-Cm-(BW)
- Inoculated (Reporter) culture from glycerol stock(-80°C freezer) in 2mL LB medium.(LB-Amp,Cm)(LB-Amp,Cm,AHL100nM)
Inoculated transformants for 12 hours in 2ml LB-Ampicillin, Chloramphenicol medium and LB-Ampicillin, Chloramphenicol, 100nmAHL medium .(37&ged;C, 12h). それぞれを105希釈してAHLが入っていないものはLB-Amp,Cm,AHLが入っているものはAHL-Amp,Cm,AHL100nMのプレートにそれぞれ20μl撒く。
37°Cで12h培養後、きちんとコロニーができていることを確認し、両方のプレートにUVを照射する。UVの波長は254nm,UVランプとプレートの距離は14cm。
UVを照射して9h後、21h後にそれぞれ両方のプレートに次の同じ操作をする。
両方のプレートのコロニーをつついてそれぞれ(LB-Amp,Cm),(LB-Amp,Cm,AHL100nM)2ml培養する。
37°C,で12h培養後、濁っていたので、それぞれを(LB-Amp,Cm),(LB-Amp,Cm,AHL100nM)のプレートに105希釈して20μl撒いた。
- Colony count
| no AHL | AHL100nM | |
| 9h | 606 | 453 |
| 12h | 146 | 151 |
Colonies of no AHL plate were AHLが入っていないほうのコロニーはいづれも光っていた。→機能チェックはOK
Team:Communication
- (20/8)--->PCR
- [http://partsregistry.org/Part:BBa_C0070 BBa_C0070](2007)
- [http://partsregistry.org/Part:BBa_C0070 BBa_C0070](2006)
- [http://partsregistry.org/Part:BBa_C0076 BBa_C0076](2007)
- [http://partsregistry.org/Part:BBa_C0078 BBa_C0078](2007)
- [http://partsregistry.org/Part:BBa_C0078 BBa_C0078](2006)
| DNA Template | 1 |
| dNTP mix | 10 |
| Foward Primer | 5 |
| Reverse Primer | 5 |
| DNA polymerase TAQ | 1 |
| Thermopol Buffer | 5 |
| dH2O | 28 |
| TOTAL | 50μL |
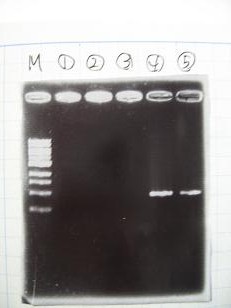
- 95°C,5min -> ( 95°C,1min -> 52°C,1min -> 72°C,1min )・・・25cycles -> 72°C,10min -> 6°C
- --->Gel Check
- Transformation
- competent cells : XL10G
- [http://partsregistry.org/Part:BBa_S03154 BBa_S03154](2007)
- [http://partsregistry.org/Part:BBa_S03154 BBa_S03154](2006)
- [http://partsregistry.org/Part:BBa_I9026 BBa_I9026](2007)
- [http://partsregistry.org/Part:BBa_I9026 BBa_I9026](2006)
- [http://partsregistry.org/Part:BBa_I9030 BBa_I9030](2007)
- [http://partsregistry.org/Part:BBa_I9030 BBa_I9030](2006)
--->(23/8)Digestion
Team:Output
- [http://partsregistry.org/Part:BBa_J32007 BBa_J32007](2007)-->no colony-->no colony(2 times)-->colony(3times)
- [http://partsregistry.org/Part:BBa_B0034 BBa_B0034](2007)-->colony
 "
"