Team:Chiba/jk/β/week 3
From 2008.igem.org
>送受信班
Contents |
Week 3
31 August, 2008
- Transformation
- Competent Cells : XL10G
- ---> We saved these plates with a refrigerator.
Sample No 1~3 4 Sample DNA 12 10 PstⅠ 0.2 0.2 XbaⅠ 0.2 - SpeⅠ - 0.4 Buffer 2 - 1.5 Buffer 3 2 - BSA 2 1.5 dH2O 3.6 1.4 TOTAL 20 15
- --->(1/9)Gel Check
- (30/8)--->Mini prep
- --->Digestion test
- Plac+RBS+RhlI Sample No.2~5
- BBa_T9002[7]①、②
Sample Single Digesiton 2~5 Double Digestion 2~5 Single Digestion T9002① Single Digesiton T9002② Double Digestion T9002①② Sample DNA 1 3 3 1 3 XbaⅠ 0.1 0.1 0.1 0.1 0.1 SpeⅠ - 0.1 - - 0.1 Buffer 2 0.9 0.8 0.9 0.9 0.8 BSA 1 1 1 1 1 dH2O 7 5 5 7 5 TOTAL 10 10 10 10 10
- --->Gel Check
1 September, 2008
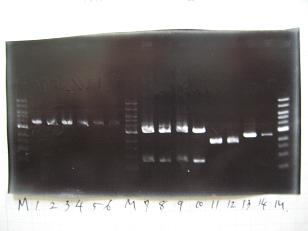
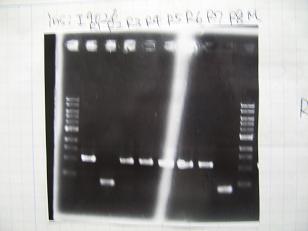
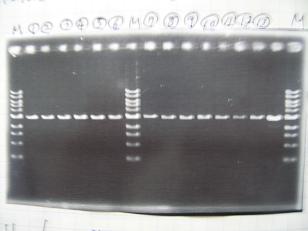
- (31/8)--->Gel Check
Sample No 1~3 4 Sample DNA 20 15 Loading Dye 4 3 TOTAL 24 18 - From left;
- insert-1(I9026)
- insert-2(I9030)
- From left;
- insert-3(S03154)
- vector-4(R0010)
- --->Gel extract
- --->zymo
- insert-1(I9026) -> 7μL
- insert-2(I9030) -> 7μL
- insert-3(S03154) -> 7μL
- vector-4(R0010) -> 15μL
- --->SAP
- vector-4(R0010)
- --->Zymo
- vector-4(R0010) -> 20μL
- --->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- insert-1(I9026) -> OK
- insert-2(I9030) -> OK
- insert-3(S03154) -> None --> Transformation BBa_S03154[12]
- vector-4(R0010) -> OK
- From left;
- --->Ligation
Sample No (1) (2) (3) (4) (5) insert-1(I9026) 3 - 3 - - insert-2(I9030) - 3 - 3 - vector-4(R0010) 3 3 - - 3 ligase 1 1 1 1 1 Buffer 1 1 1 1 1 dH2O 2 2 5 5 5 TOTAL 10 10 10 10 10
- --->Transformation
- Competent cells : XL10GOLD 30μL
- Transformed the following and grew on new ampicillin plates.
- --->(2/9) Colony PCR
- Competent cells : BW ΔFliC 40μL
- Transformed the following and grew on new ampicillin plates.
- --->(2/9)Liquid Culture
2 September, 2008
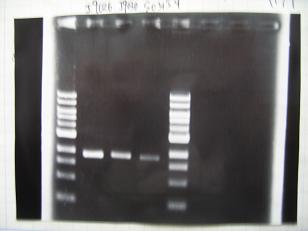
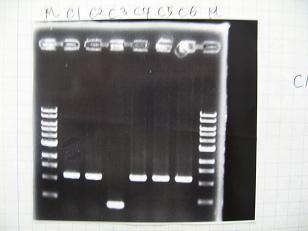
- (31/8)--->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- I9026 -> OK, 100/μL
- I9030 -> OK, 50ng/μL
- S03154 -> OK, 30ng/μL (too low for the ligation:1/9 )
- From left;
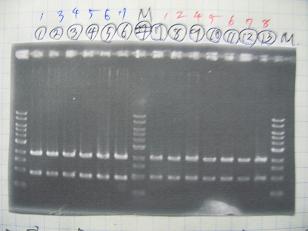
- (1/9)---> Colony PCR
- Colony PCR of 8 colonies from ligation plates (1/9-(1),(2)) and one from control plate(BBa_F2620[18](2007)).
DNA Template 1 dNTP mix 5 Foward Primer 0.3 Reverse Primer 0.3 DNA polymerase TAQ 0.5 Thermopol Buffer 3 dH2O 20.5 TOTAL 30μL
- 95℃,5min -> ( 95℃,1min -> 52℃,1min -> 72℃,1min )・・・25cycles -> 72℃,10min -> 6℃
--->Gel CheckSample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- Plac+RBS+RhlI+LVA
- R1 -> OK
- R2 -> Bad
- R3~R7 -> OK
- R8 -> Bad
- From left;
- From left;
- Plac+RBS+CinI+LVA
- C1,C2 -> OK
- C3 -> Bad
- C4~C6 -> OK
- From left;
- Plac+RBS+CinI+LVA
- C7,C8 -> OK
- BBa_F2620[19](2007):Positive control -> OK
- --->(3/9)Mini prep
- (1/9)--->Liquid Culture
- Cultured the following cells (2mL LB-Amp, at 37℃, 7 hours)
- --->(3/9)Phenotype test
- --->(4/9)Mini prep
3 September, 2008
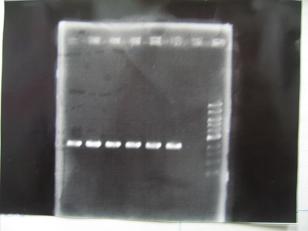
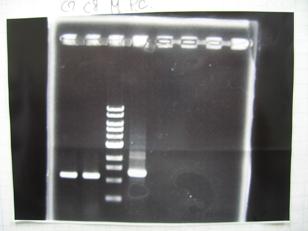
- (2/9)--->Mini prep
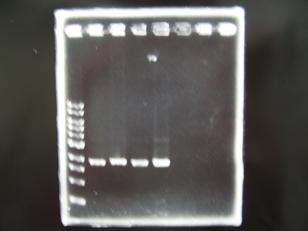
- --->Gel Check
Sample DNA 1 Loading Dye 1 dH2O 4 TOTAL 6 - From left;
- S03154 -> OK
- R1,R3~R7 (Plac+RBS+RhlI+LVA) -> OK
- From left;
- From left;
- C1,C2,C4~C8 (Plac+RBS+CinI+LVA) -> OK
- --->Digestion test
- R1,R3~R7 (Plac+RBS+RhlI+LVA)
- C1,C2,C4~C8 (Plac+RBS+CinI+LVA)
Digestion Single Double Sample DNA 1 3 XbaⅠ 0.1 0.1 PstⅠ 0.1 0.1 Buffer 2 0.9 - Buffer 3 - -0.8 BSA 1 1 dH2O 7 5 TOTAL 10 10
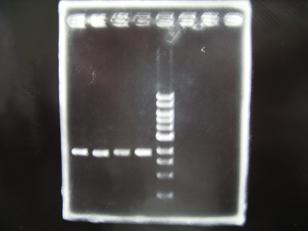
- --->Gel Check
Sample DNA 10 Loading Dye 2 TOTAL 12 - From left;
- Single Digestion : R1,R3~R7 -> OK
- Single Digestion : C1,C2,C4~C8 -> OK
- From left;
- From left;
- Double Digestion : R1,R3~R7 -> OK
- Double Digestion : C1,C2,C4~C8 -> OK
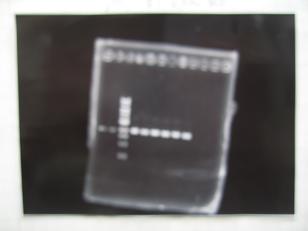
- (2/9)--->Phenotype-test
- MIX
Sample No. 1 2 3 4 5 6 7 8 9 10 11 L1 2 - - - - - - - - - - L2 - 2 - - - - - - - - - L3 - - 2 - - - - - - - - L4 - - - 2 - - - - - - - R1 - - - - 1 - - - - - - R2 - - - - - 1 - - - - - R3 - - - - - - 1 - - - - R4 - - - - - - - 1 - - - BBa_S03154[32] - - - - - - - - 2 - - BBa_T9002[33] 2 2 2 2 1 1 1 1 2 1 1 AHL(100μM) 1 1 1 1 1 1 1 1 1 1 1 - Incubated for 8hours at 37 degrees
- Spindown (max rpm, 3 min)
- The measurement of the intensity GFP by Masahiro Tominaga
Sample No 1 2 3 4 5 6 7 8 9 10 11 the intensity GFP + + + + + + + ++ + - +++ 4 September, 2008
- --->Gel Check
Sample No ① ② ③ ④ Sample DNA 3 3 3 3 Loading Dye 2 2 2 2 dH2O 7 7 7 7 TOTAL 12 12 12 12 - From left;
Sample No ① ② ③ ④ Sample DNA 3 3 3 3 Loading Dye 2 2 2 2 dH2O 7 7 7 7 TOTAL 12 12 12 12 - From left;
Sample No ① ② ③ ④ Sample DNA 3 3 3 3 Loading Dye 2 2 2 2 dH2O 7 7 7 7 TOTAL 12 12 12 12 - From left;
5 September, 2008
6 September, 2008
>next week
ホーム メンバー紹介 プロジェクト紹介 Parts Submitted to the Registry モデリング ノート
 "
"