Team:Chiba/jk/γ/Trl
From 2008.igem.org
(Difference between revisions)
(→Protocol) |
(→装置&試薬(apparatus&reagents)) |
||
| Line 9: | Line 9: | ||
===装置&試薬(apparatus&reagents)=== | ===装置&試薬(apparatus&reagents)=== | ||
| - | + | *Equipment | |
| - | + | :shaking incubator | |
| - | + | ::Innova 4200 Benchtop or Floor-Stackable Incubator Shaker(37°C) | |
| - | + | :46-well plate(deep well) | |
| + | :96-well plate(deep well) | ||
| + | :Fluoroskan Ascent 2.5(program:Ascent Software Version 2.6) | ||
| + | :Beckman Allegratm X-12R Centrifuga(Beckman Coulter) | ||
| + | |||
| + | |||
'''試薬(reagents)''' | '''試薬(reagents)''' | ||
*AHL(100uM,5uM,100nM) | *AHL(100uM,5uM,100nM) | ||
Revision as of 23:52, 29 October 2008
Contents |
Time Responce Liquid
purpose
インダクションをかけてからいち早く、確認できる出力を見つけること
To find the earliest output gene after induction
装置&試薬(apparatus&reagents)
- Equipment
- shaking incubator
- Innova 4200 Benchtop or Floor-Stackable Incubator Shaker(37°C)
- 46-well plate(deep well)
- 96-well plate(deep well)
- Fluoroskan Ascent 2.5(program:Ascent Software Version 2.6)
- Beckman Allegratm X-12R Centrifuga(Beckman Coulter)
試薬(reagents)
- AHL(100uM,5uM,100nM)
- IPTG(100nM)
- X-gal
- M9
- PBS
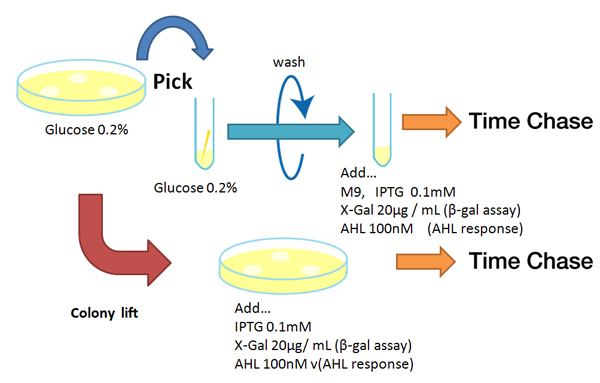
Method
Strain:XL10G KanR
- Liquid medium experiment
- Pre-culture
- Picked and cultured the following plate in 2mL of LB:
- LB-Amp+0.2 % Glucose, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- LB-Amp, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Cultured at 37°C for 12h.
- Picked and cultured the following plate in 2mL of LB:
- Culture
- Dilute pre-cultures and add to new LB medium.
- LB-Amp+0.2 % Glucose, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- LB-Amp, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Cultured at 37°C for about 6 h
- Dilute pre-cultures and add to new LB medium.
- Wash
- Transfer 10mL each of the culture to 50mL centrifuge tubes.
- Centrifuged for 6min at 3600rpm,20°C and discarded the supernatant.
- Add physiological saline and resuspention
- Repeated wash twice.
- Add M9 minimal medium.
- Mix
- Dispens each culture into 48-well deep well or 96-well deep well
- Add IPTG fainal conc. 0.2 mM (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- Add AHL fainal conc. 100nM ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Measure fluorescence intensity every 1 h.
Result
| ホーム | メンバー紹介 | プロジェクト紹介 | Parts Submitted to the Registry | モデリング | ノート |
|---|
 "
"