Team:Chiba/jk/γ/trs
From 2008.igem.org
(Difference between revisions)
(→Time Responce Liquid) |
(→Discussion) |
||
| Line 46: | Line 46: | ||
| - | + | '''Discussion''' | |
| - | + | Discussion | |
| - | |||
| - | |||
| - | |||
| - | + | The purpose of this project is to alter the time required for expression. | |
| - | * | + | * By increasing the concentration of X-gal, the time required for |
| - | + | expression to be observable decreased, but this still takes longer | |
| + | than the time required when using fluorescent proteins. | ||
| + | |||
| + | *Using β-gal, which uses X-gal as substrate, the time required for | ||
| + | output may increase because the substrate concentration decreases with | ||
| + | time. | ||
| + | |||
| + | For the above reasons, fluorescence proteins are more suited for our purposes. | ||
| + | |||
| + | Of the fluorescent proteins, GFP and YFP fluorescence was observable | ||
| + | at earlier stages. | ||
| + | |||
| + | |||
| + | * But to the naked eye, GFP could be observed more easily. | ||
| + | |||
| + | Therefore, we chose GFP as our output reporter. | ||
Revision as of 04:40, 30 October 2008
Contents |
Time Responce Liquid
Reporter
- Fluorescent Protein
- GFP
- pGFPuv
- BBa_T9002
- Venus YFP
- BBa_K084003
- pLac-Venus YFP
- mCherry
pLac-mCherry
- β-gal (X-gal assay)
- pUC19(plac-LacZα)
Equipment
- shaking incubator
- Innova 4200 Benchtop or Floor-Stackable Incubator Shaker(37°C)
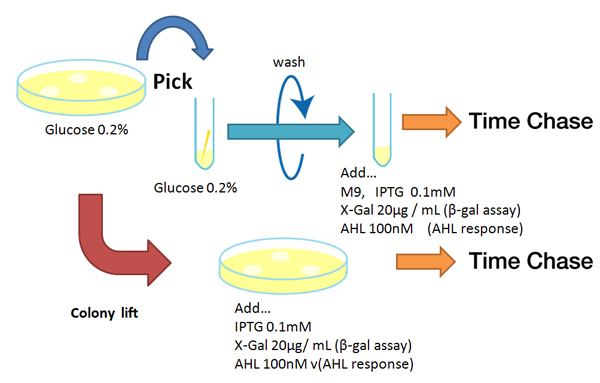
Method
Strain:XL10G KanR
- Agar plate experiment
- Pre-culture
- Picked and cultured the following plate stocks in 2mL of LB:
- LB-Amp+0.2 % Glucose, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- LB-Amp, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Cultured at 37°C for 12h.
- Picked and cultured the following plate stocks in 2mL of LB:
- Spread on plate
- Spread on new plate
- LB-Amp+0.2 % Glucose, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- LB-Amp, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Cultured at 37°C for 12h.
- Spread on new plate
- Colony lift
- Colony lift to inducible agar plate (containing IPTG or AHL)
- LB-Amp+0.2 mM IPTG agar plate, (pGFPuv, pLac-Venus YFP, pLac-mCherry, pUC19)
- LB-Amp+100 nM AHL agar plate, ([http://partsregistry.org/Part:BBa_T9002 BBa_T9002], [http://partsregistry.org/Part:BBa_K084003 BBa_K084003])
- Incubate at 37 °C
- Colony lift to inducible agar plate (containing IPTG or AHL)
- Check expression every 30 min.
Result
Discussion
Discussion
The purpose of this project is to alter the time required for expression.
- By increasing the concentration of X-gal, the time required for
expression to be observable decreased, but this still takes longer than the time required when using fluorescent proteins.
- Using β-gal, which uses X-gal as substrate, the time required for
output may increase because the substrate concentration decreases with time.
For the above reasons, fluorescence proteins are more suited for our purposes.
Of the fluorescent proteins, GFP and YFP fluorescence was observable at earlier stages.
- But to the naked eye, GFP could be observed more easily.
Therefore, we chose GFP as our output reporter.
| ホーム | メンバー紹介 | プロジェクト紹介 | Parts Submitted to the Registry | モデリング | ノート |
|---|
 "
"