Team:Edinburgh/Plan
From 2008.igem.org
(→Glycogen overproduction) |
(→Starch biosynthesis) |
||
| Line 58: | Line 58: | ||
* Wattebled F et al. 2005. Mutants of Arabidopsis lacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin. Plant Physiology '''138''', 184-195. | * Wattebled F et al. 2005. Mutants of Arabidopsis lacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin. Plant Physiology '''138''', 184-195. | ||
| - | * Kubo A et al. 2005. Complementation of sugary-1 phenotype in rice endosperm with the wheat isoamylase1 gene in supports a direct role for isoamylase1 amylopectin biosynthesis. Plant Physiology '''137''', 45-36. | + | * Kubo A et al. 2005. Complementation of sugary-1 phenotype in rice endosperm with the wheat isoamylase1 in gene in supports a direct role for isoamylase1 amylopectin biosynthesis. Plant Physiology '''137''', 45-36. |
===Synthesis of carotenoids=== | ===Synthesis of carotenoids=== | ||
Revision as of 11:32, 28 October 2008
Contents |
A chassis: E. coli vs. B. subtilis
We were initially torn between using Escherichia coli or Bacillus subtilis as a chassis for development of our system:
We decided that B. subtilis would have advantages in the case of our system working and industrialisation being an option. This was because of increased ease of transport (due to its stable spore-forming nature), its having a better characterised secretory system and being regarded in higher favour than E. coli by the general public (see section of ethical, legal and social implications). Another possibility for the future would be to port the system to yeast, Saccharomyces cerevisiae; this would be more difficult to achieve but might ultimately be more desirable as yeast is Generally Regarded As Safe for food use, and as a eukaryote, might be able to accumulate larger amounts of starch than bacteria.
However, for the labwork we decided to use E. coli JM109 as the chassis. This was due to the greater availability of tested registry parts for E. coli compared to B. subtilis, a higher level of understanding of the biochemistry of E. coli, and more in-house experience of engineering E. coli.
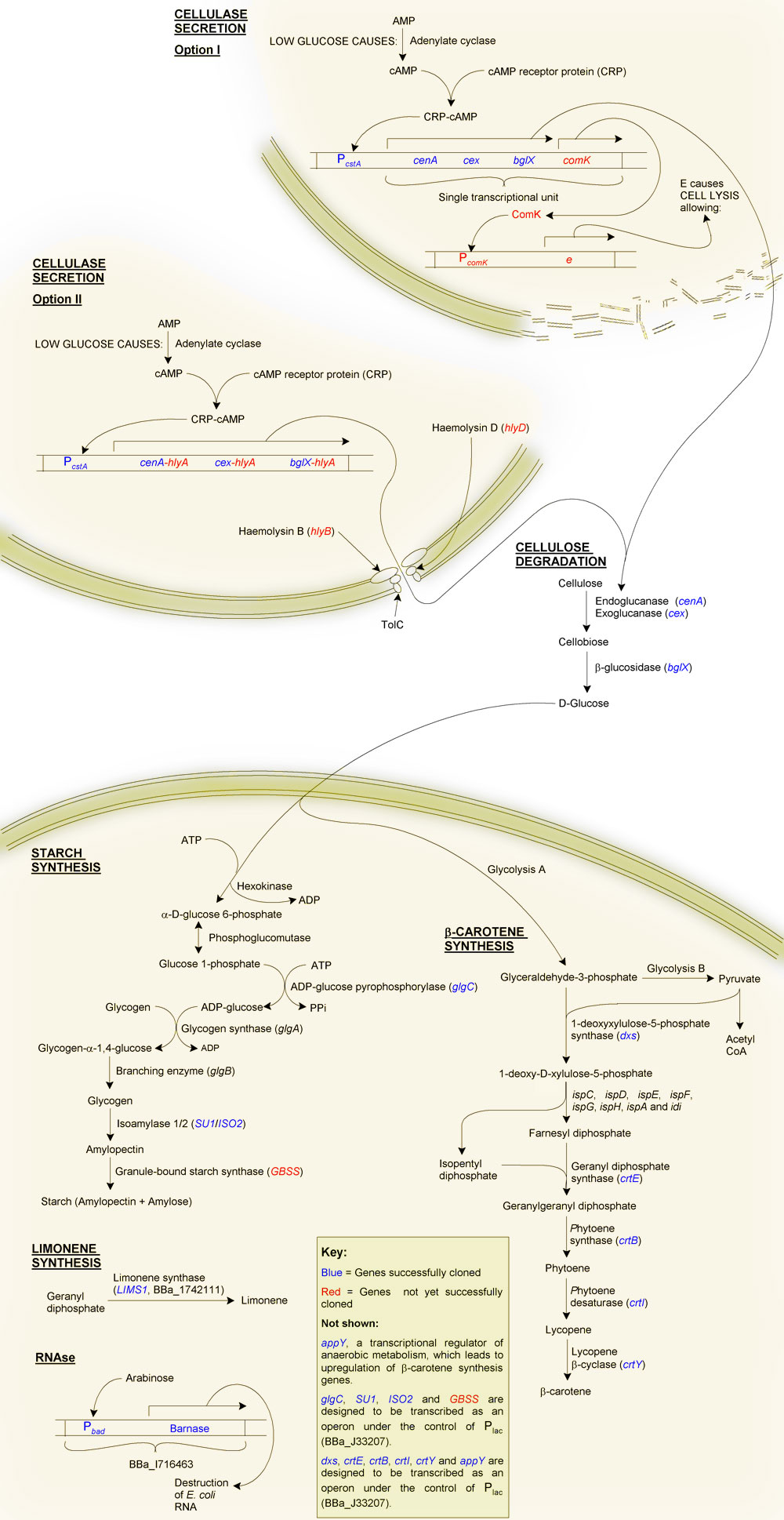
Primary Objective: The production of starch from cellulose
Firstly, why starch?
Although one of our aims is to provide a substrate for the biofuels industry, which can easily ferment glucose to ethanol, we decided to try to synthesise starch instead, even though this requires extra steps. The reason for this was a question of purification: glucose is soluble, and therefore difficult to purify from solution, as is glycogen. Starch on the other hand is highly insoluble making purification of this end product relatively straight forward. As well as this, glucose becomes inhibitory at high cellular concentrations. Starch, being insoluble, can accumulate at much higher quantities without disrupting the biochemistry of the cell.
The production of starch from cellulose requires two main steps:
- Cellulolysis
- Starch synthesis: The glucose must be taken up by the cell and synthesised into glycogen, and then starch.
2. Starch synthesis
The first phase in synthesising starch make use of the chasis' native glycogen synthesis pathway. The gene glgC (ADP-glucose pyrophosphorylase, catalysing the convertion glucose 1-phosphate and ATP to ADP-glucose and PPi) is responsible for the most rate-limiting step of glycogen synthesis in E. coli. This is because the protein is negatively regulated by PPi. Carrying out the substitution 336:Gly->Asp to glgC has been reported to increase the yield of glycogen due to the loss allosteric inhibition. This mutated form of glgC is one of the BioBricks which we are in the process of making.
The second phase is the conversion of glycogen to starch. To achieve this we are creating isoamylase and granule-bound starch synthesase BioBricks (isa1, isa2 and gbss) from Zea mays cDNAs. These three genes together should be sufficient for the production of starch from glycogen.
3. Beta-carotene synthesis
Vitamin A deficiency results in night-blindness and an impaired immune system. A number of genes from Pantoea ananatis will be added to our bacterial cells, in essence transferring the beta-carotene synthesis pathway of P. ananatis to our chasis organism. These genes are crtE (geranyl diphosphate synthesase), crtB (phytoene synthase), crtI (phytoene desaturase) and crtY (lycopene beta-cyclase). We will also create BioBricks of the E. coli genes dxs and appY, the addition of which should increase the yield of beta-carotene.
System overview
References
Cellulose degradation
- Lynd, L.R., Weimer, P.J., van Zyl, W.H., and Pretorius, I.S. 2002. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiology and Molecular Biology Reviews 66, 506-577.
Glycogen overproduction
- Leung, P., Lee, Y.M., Greenberg, E., Esch, K., Boylan, S., and Preiss, J. 1986. Cloning and expression of the Escherichia coli glgC gene from a mutant containing an ADP-glucose pyrophsophorylase with altered allosteric properties. Journal of Bacteriology 167, 82-88.
- Eydallin, G., Viale, A.M., Moran-Zorzano, M.T., Munoz, F.J., Montero, M., Baroja-Fernandez, E., and Pozueta-Romero, J. 2007. Genome-wide screening of genes affecting glycogen metabolism in Escherichia coli K-12. FEBS Letters 581, 2947-2953.
- Dedhia, N., Chen, W., and Bailey, J.E. 1996. Design of expression systems for metabolic engineering: coordinated synthesis and degradation of glycogen. Biotechnology and Bioengineering 55, 419-426.
- Fernandez-Banares, I., Clotet, J., Arino, J., and Guinovart, J.J. 1991. Glycogen hyperaccumulation in Saccharomyces cerevisiae RAS2 mutant - a biochemical study. FEBS Letters 290, 38-42.
Starch biosynthesis
- Ball, S.G. and Morell, M.K. 2003. From bacterial glyogen to starch: understanding the biogenesis of the plant starch granule. Annual Reveiews in Plant Biology 54, 207-233.
- Delatte T et al. 2005. Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant Journal 41, 815-830.
- Wattebled F et al. 2005. Mutants of Arabidopsis lacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin. Plant Physiology 138, 184-195.
- Kubo A et al. 2005. Complementation of sugary-1 phenotype in rice endosperm with the wheat isoamylase1 in gene in supports a direct role for isoamylase1 amylopectin biosynthesis. Plant Physiology 137, 45-36.
Synthesis of carotenoids
- Sandmann, G. 2006. Production of carotenoids by gene combination in Escherichia coli. pages 143-153 in 'Food Biotechnology', 2nd edition, eds. K. Shetty, G. Paliyath, A. Pometto and R.E. Levin, CRC Press, Taylor & Francis group, Boca Raton, FL.
- Misawa, N., Nakagawa, N., Kobayashi, K., Yamano, S., Nakamura, K., and Harashima, K. 1990. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. Journal of Bacteriology 172, 6704-612.
Synthesis of limonene and other terpenes
- Lucker, J., El Tamer, M.K., Schwab, W., Verstappen, F.W.A., van der Plas, L.H.W., Bouwmeester, H.J., and Verhoeven, H.H. 2002. Monoterpene biosynthesis in lemon (Citrus limon). European Journal of Biocehmistry 269, 3160-3171.
- Reiling, K.K., Yoshikuni, Y., Martin, V.J.J., Newman, J., Bohlmann, J., and Keasling, J.D. 2004. Mono and diterpene production in Escherichia coli. Biotechnology and Bioengineering 87, 200-212.
 "
"