Team:Edinburgh/Project
From 2008.igem.org
(→Our Objective: Saving the World) |
|||
| Line 288: | Line 288: | ||
</html> | </html> | ||
| - | For more information, please see | + | For more information, please see [https://www.wiki.ed.ac.uk/display/iGEM2008/HOME our internal wiki]. |
==Our Objective: Saving the World== | ==Our Objective: Saving the World== | ||
| Line 300: | Line 300: | ||
Currently, much agricultural produce is wasted. Wouldn't it be great if the indigestible parts of crop plants could be made edible, or at least if they could be converted into a biofuel source? - This is our plan! | Currently, much agricultural produce is wasted. Wouldn't it be great if the indigestible parts of crop plants could be made edible, or at least if they could be converted into a biofuel source? - This is our plan! | ||
| - | We will engineer | + | We will engineer bacteria with three novel pathways: |
# Degrade cellulose into glucose | # Degrade cellulose into glucose | ||
# Synthesise starch from glucose | # Synthesise starch from glucose | ||
Revision as of 19:07, 31 July 2008
For more information, please see our internal wiki.
Contents |
Our Objective: Saving the World
"Cellulose is the most abundant form of fixed carbon, with 100,000,000,000 tons produced in cell walls by plants each year" [http://www.blackwell-synergy.com/doi/full/10.1196/annals.1419.026 (Wilson, 2008)].
A food shortage is engulfing the entire world, pushing food prices to new highs. An essay from the [http://www.earthpolicy.org/Updates/2008/Update72.htm Earth Policy Institute] (16 April 2008) discusses its significance to our lives. The food shortage not only harms the economy but also overburdens the environment, putting the future of the human race in peril. Coupled to this is global warming resulting in more frequent and lengthier periods of drought for much of the world as well as predicted inundation of low-lying land, and the depletion of oil reserves, which together but further strain on ecosystems.
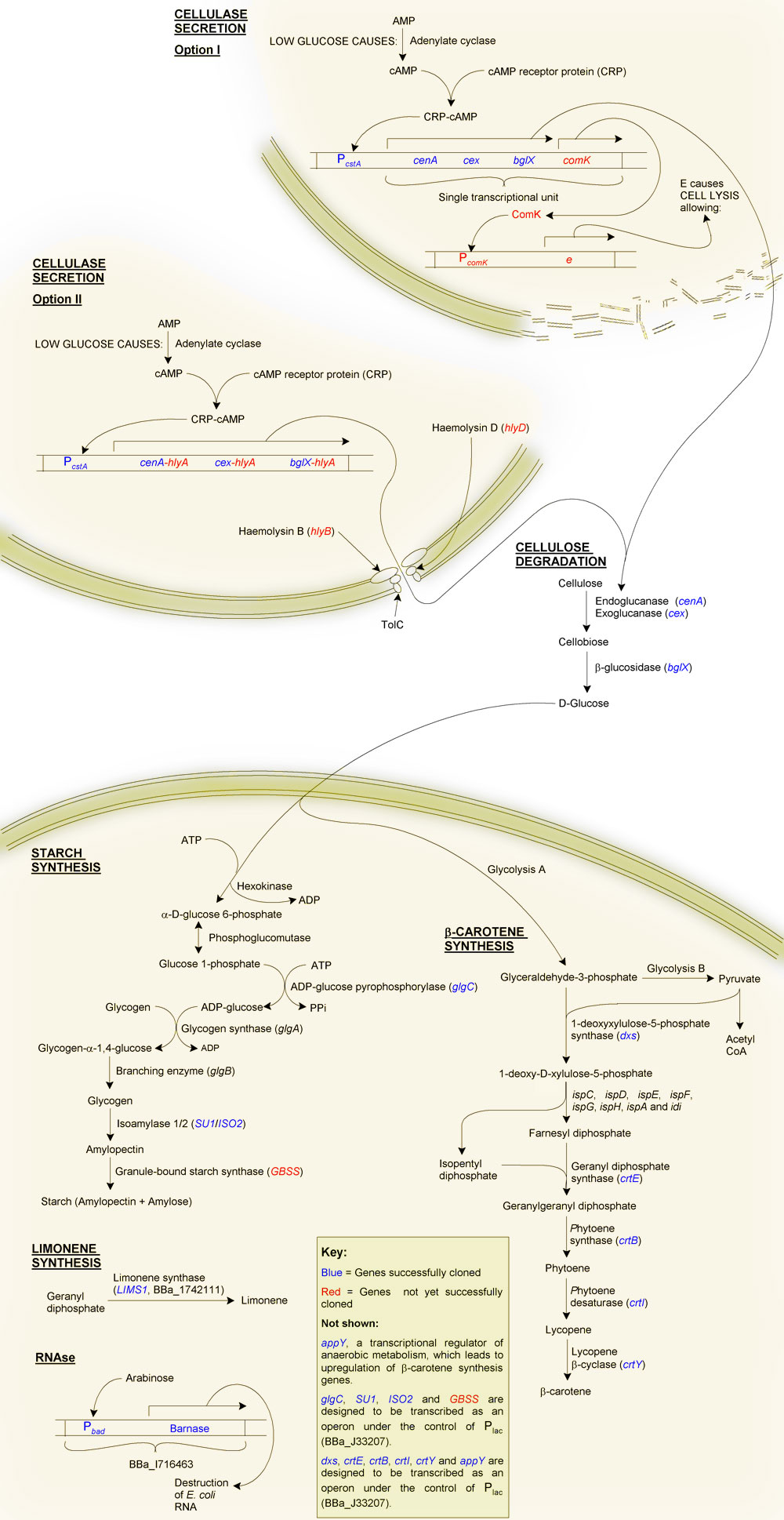
Project Description: Engineering bacteria to convert cellulose into starch and to produce the vitamin A precursor, beta-carotene
Currently, much agricultural produce is wasted. Wouldn't it be great if the indigestible parts of crop plants could be made edible, or at least if they could be converted into a biofuel source? - This is our plan!
We will engineer bacteria with three novel pathways:
- Degrade cellulose into glucose
- Synthesise starch from glucose
- Synthesise beta-carotene from glucose
1. Cellulose degradation
We plan to incorporate the Cellulomonas fimi endoglucanase genes cenA, cenB and cenC, exoglucanase gene cex and beta-glucosidase into bacteria. Together these genes will be capable of breaking down cellulose into glucose. The proteins will need to be secreted into the medium to act (cellulose being too large for uptake into the cells), and we have 3 plans to enable this:
- Add the Hly secretory pathway into a lab strain of E. coli (K12, JM109). This will involve incorporating hlyB, hlyD and tolC and adding the 3' end of hlyA to our cellulases.
- Create a glucose-sensitive feedback mechanism of cell lysis. Depletion of glucose would cause transcription of our cellulase operon and the transcription factor comK. ComK would bind to a promoter upstream of the phiX174 gene E. E is a short peptide which causes cell lysis.
- Engineer our genes into Bacillus subtilis, in which secretion is much better understand than in E. coli.
2. Starch synthesis
This would make use of the native glycogen synthesis pathway. We have identified a mutation in the gene glgC which should result in increased yield of glycogen. Adding isoamylase and granule-bound starch synthesis genes from Zea mays should then allow the cells to convert the glycogen into starch.
3. Beta-carotene synthesis
Vitamin A deficiency results in night-blindness and an impaired immune system. A number of genes from Pantoea ananatis will be added to our bacterial cells, in essence transferring the beta-carotene synthesis pathway of P. ananatis to our chasis organism. These genes are crtE (geranyl diphosphate synthesase), crtB (phytoene synthase), crtI (phytoene desaturase) and crtY (lycopene beta-cyclase). We will also create BioBricks of the E. coli genes dxs and appY, the addition of which should increase the yield of beta-carotene.
 "
"