Team:Heidelberg/Human Practice/Phips the Phage/Technical Backround
From 2008.igem.org


Technical Background Information
Molecular Cloning
Molecular cloning is a procedure to isolate a defined DNA sequence and obtain multiple copies of it in vivo - which means in a living cell, in our case a bacterial cell. So the term cloning refers to the amplifying of a DNA sequence, but it is also often used to describe the procedure of engineering a given DNA sequence and thereby altering it. This is not far fetched, because this engineering procedure also contains an essential amplifying step. Cloning is utilized in a wide array of biological experiments, and it is also one of the most important techniques in biotechnology, and also still in synthetic biology. We constantly used molecular cloning in our project.
What I want to explain to you in more detail here is how a given DNA sequence is altered in order to encode for new properties. In most cases proteins are responsible for certain properties (see Gene and Proteins), and therefore the DNA sequence of genes encoding proteins is altered, added or removed from the engineered DNA sequence.
So let me describe this procedure in greater detail, explaining the example of how to get the information for a fluorescent protein into a bacterial plasmid.
You ask yourself what a fluorescent protein is? Well, it is a protein, which is able to absorb light and emit light of lower energy and thereby glow. One protein with this feature is taken from a jellyfish and called Green Fluorescent Protein (GFP). It is the oldest and most broadly used fluorescent protein in biotechnology. Because it had such a great impact on this science and improved it a lot, this year’s Nobel Prize in chemistry was awarded for the discovery of GFP and its applications. If GFP is made by a cell and you illuminate this cell with UV-light, you can see a green light coming from this cell.
So imagine you made the discovery of GFP and found the sequence in the genome of this jellyfish, which encodes for the protein. Now you want this protein to be produced in a bacterial cell, so you can visualize it as described above. How do you proceed? You have to isolate the DNA encoding the GFP (the insert) and clone it into a bacterial plasmid (the vector), so that it is amplified and expressed.
Get the DNA
How do we get the DNA? There are three major possibilities: Cut it out of the original DNA, replicate it via PCR (Polymerase Chain Reaction) or synthesize it from scratch.
Restriction enzymes
Naturally there exist proteins, which are able to cut DNA. They are called restriction enzymes. They work by cutting the bonds between two nucleotides of the DNA strands and thereby create two new ends of the double helix. But restriction enzymes do not cut the DNA at random points, but they need signal sequences to get active. Those are called restriction sites and only at those sites the enzymes can bind and cut the DNA. So restriction enzymes provide a tool to cut DNA sequences in defined locations. Organism derived DNA sequences have a great number and variety of restriction sites. Over 3000 restriction enzymes have been studied in detail, and more than 600 of these are available commercially and are routinely used for DNA modification and manipulation in laboratories. The possibility to find suitable restriction sites to excise a certain part of DNA is generally very high.
Polymerase Chain Reaction
This technique uses enzymes which are called DNA-polymerases. They are able to amplify a piece of DNA (like the polymerases that amplify our genome if a cell divides). They do this so called DNA replication in putting complementary nucleotides to a single DNA strand, thereby complementing it. If a DNA double strand is separated into two single ones, the DNA polymerase can complement the single ones and make them into double strands again. If they are separated anew (now we are having four single strands), the polymerase will again complement them, and so on. By constantly separating double stranded DNA and complementing them with the help of DNA polymerases, you can exponentially increase the amount of the given DNA fragment. Because the newly made DNA strands are serving as templates for the next amplifying circle, the reaction is called a ‘chain reaction’.
But one thing is very important to know: DNA polymerases need a starting point for the complementation. They need a small part of double stranded DNA, where they can start to add nucleotides to. Genetic engineers provide this starting point in the form of so called primers: These are small pieces of DNA which are complementary to parts of the DNA-strands to amplify. So they form small sections of double stranded DNA. By taking primers, that flank the DNA sequence which carries the gene of interest, the DNA polymerase will only amplify this flanked sequence. So by adding the right primers to the reaction, you can get the insert you need. Naturally, to take the right primers you need to know parts of or the whole sequence of the gene of interest. With the help of the primers you can also add restriction sites at the ends of the prospective insert. They will be important in a later step.
The last question you may ask is: How are the DNA strands separated? This is done by heating the DNA. At high temperatures (>70°C) the bonds between the bases that hold together the double strand are loosened and the strands separate. Right, now you have to ask the real last question? How can enzymes survive these temperatures and not denature like most proteins? The DNA polymerases taken for this reaction originate from Bacteria which live in very hot environments like thermal springs. Therefore they evolved enzymes which are not only able to survive high temperatures, but also work very efficiently.
To sum this technique: DNA strands are amplified by many cycles of heat separation (or denaturising) DNA double strands into single ones and subsequently complementing these single strands via a DNA polymerase.
Synthesis from scratch
It is also possible to synthesise the insert with the gene of interest from scratch out of nucleotides. Therefore you have to know the complete sequence you want to make. You also have to add restriction sites at the end.
So now we have the insert with the gene of interest, in our case the GFP, how do we get this into the bacterial vector? From all I have told you, you can make an elaborate guess!
Right, we can again use restriction enzymes to cut the vector open and have to new DNA ends in the vector. All we have left to do is to link these ends to the two ends of the insert and we have put the insert into the vector. But how is this done?
Ligation
Therefore I have to explain another characteristic of restriction enzymes to you: The ends of the DNA, which have been cut by restriction enzymes, have a very important property: They are able to bind to other DNA ends, which have been cut by the same enzyme. You can imagine this to be because restriction enzymes cut a certain pattern into the DNA ends. Do you remember scissors which cut different patterns, like zigzag or waves into paper from when you where young? Restriction enzymes figuratively work like this: Only DNA ends, which have been created by the cut of the ‘zigzag enzyme’, have a zigzag pattern and can therefore bind to each other.
So if you cut your vector with a ‘zigzag enzyme’ and your insert has also been cut with a ‘zigzag enzyme’, the ends can bind to each other and the insert can thereby be put into the vector. This is why it is very important to have the right restriction sites at the ends of your insert.
The last step which is needed is to fix the bonds between the ends of the insert and of the vector, so they won’t open up again. An enzyme called Ligase is able to do this.
Now we have the vector with the insert ready. The next step of molecular cloning is to get this vector into bacterial cells, so they can amplify it and produce the protein of interest (GFP).
Transformation
This is how the procedure of getting a plasmid vector into a bacterial cell is called. There are various techniques to obtain this aim, and they all have a common strategy: The cell wall and plasma membrane of the bacterial cells are temporarily perforated – this can be done via electric pulses or high concentrations of specific salts. The results are always the same. Through the holes in the bacterial cell walls, the plasmids which are given into the medium with the bacteria can enter into the bacterial cells.
Now, all that has to be done is to let the bacteria grow and amplify the plasmids. Well, its almost all that has to be done. There is one last important point. Since the efficiency of the transformation is very low, only few of the bacterial cells will grow have the plasmid with the GFP gene. To find these few positives again, you have to apply a selection method to these from those not successfully transformed.
Selection
In our case this is very easy: You can just watch your cells illuminated with UV-light and you will see which ones have the GFP protein, for they will glow green. If you are not as lucky as we are and have cloned a gene of interest, which cannot be visualized so easy, you have to use other selection methods. Generally, the transformed cells are selected via antibiotic resistance: Therefore an antibiotic resistance gene is added to the bacterial vector, which enables the transformed bacteria to survive, if antibiotics are present in the culture media. All untransformed cells die due to the added antibiotics, because they do not have resistant genes, which neutralize the deathly power of the antibiotics.
After the selection and growing of the transformed cell, the plasmids with the gene of interest can be isolated via minipreparation and checked via Agarose gel electrophoresis. With the isolated plasmids, a new round of engineering could start, too.
Minipreparation
After being given a theoritcal introduction, the pupils carry out a plasmid minipreparation using a standard kit.
Theoritcal background:
All synthetic biologists in the lab need plasmids as very important tools for their work. You can find them in bacterial cells – either naturally, or put in there by scientists. The bacteria amplify the plasmids in their cells (they replicate them, meaning they make identical copies) and of course they multiply the total amount of plasmids by growing – more bacteria means more plasmids on the whole. If we want to work with these plasmids, like checking whether they are the right ones or engineering them, we need to get them out of the bacteria. Therefore we need plasmid preparation.
The procedure is simple, but honestly, it is not very convenient for the bacteria: We need to destroy them; scientists say to lyse them, to set the plasmids free together with the other contents of the cell. Then you will have a mixture of DNA (chromosome and plasmids), RNA, proteins and others cell components. But you only need the plasmid DNA in this case, everything else has to be separated from it, because the rest can have a negative effect on further applications.
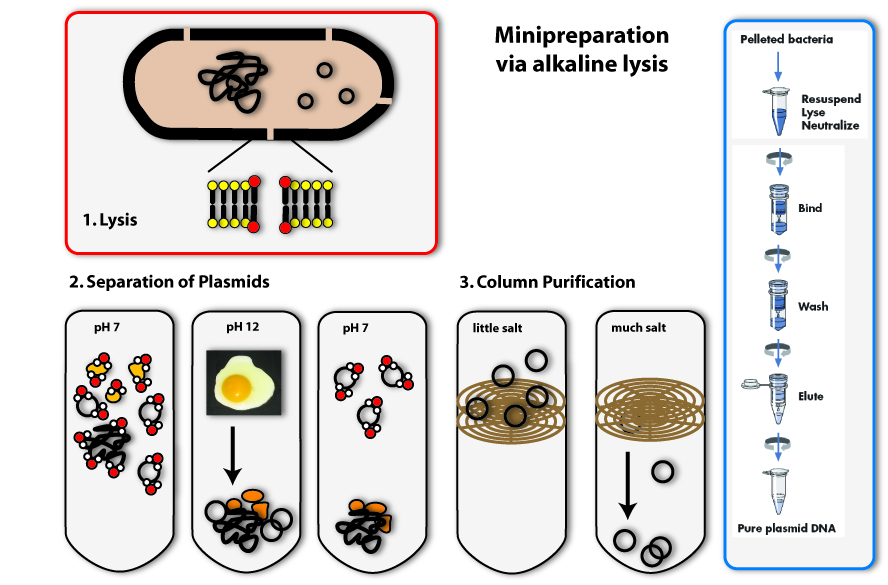
The method we mostly use in the laboratory to isolate plasmids is called alkaline lysis. This classical method is today standardized. Like the name implicates, you need alkaline agents for the lysis of the cell. Additionally, you use detergents, to break the cell wall and lyse the cells.
1. Lysis
You can, for example, also find detergents in washing agents. There they help to remove grease spots. This works as follows: Detergents have a very similar structure to lipids. They therefore can mix with them and loose their connection to fibres of the clothes. In Bacteria, they act in a very similar way: They mix with the lipids that form the bilayer of the plasma membrane and destroy there ordered structure. This is how they make holes in the plasma membrane, which leads to the lysis of the cell.
2. Precipitation and separation of proteins and chromosomal DNA
Now we come to the alkaline part of this procedure: In adding sodium hydroxide (NaOH) to the solution, the pH is increased from roughly 7 to 12. Because this is a huge contrast to the normal physiological pH, proteins and DNA denature and change their conformation. RNA is degraded. Proteins and DNA, which are denatured cannot be soluted any more and precipitate. You all know the phenomenon of denatured proteins from making fried eggs: The egg changes its consistency if you heat it and gets hard – this is due to the denaturising proteins. The same effect as heat, have acids or bases (like NaOH). In denatured DNA, the two strands get separated from each other.
The trick the alkaline method uses, to separate the plasmid DNA from the chromosomal DNA and the proteins, is to lower the pH again by adding acid (for example potassium acetate) to the solution. If the pH approximates the physiological one, the plasmids are able to renature first, because they are small and the two DNA strands find each other with a much higher possibility than the ones of the big chromosome. Therefore if you keep the renaturising step short, only the plasmids are able to build double helices and solute again. The chromosomal DNA and the proteins stay precipitated. They can be separated from the plasmids by centrifugation. (The white solids on the tube after centrifugation are the precipitated proteins, DNA and other cell components. The plasmid DNA is soluted in the clear supernatant.)
3. Binding of DNA
Now how do we get the plasmids out of this salty solution (remember we put a lot of salts to the solution to first rise, then lower the pH) into a clear one that can be used for further applications? You could precipitate the plasmids like we did with the proteins before, but there is another possibility, which is widely used today: You bind the DNA to a solid phase. This often consists of silica, which can be used in a bead like form, but in most cases, it is included in a column integrated in an eppendorf tube. The DNA can bind to the silica column, if the solution is very salty. If you centrifuge, the salty solution is drawn through the column into the tip of the tube and the DNA remains bound to the column.
4. Eluation of the plasmids
In the last step, you eluate the DNA from the column, meaning you loosen the bonds between DNA and silica and solute the DNA again. This is possible with every solution containing low or no salt at all. You can take water, for example. You put it on the column, centrifuge again, but this time the DNA will also be drawn through the column into the tip of the tube together with the water. You can then proceed to work with your purified plasmids: you can, for example, check, whether you have isolated the right one using agarose gel electrophoresis.
 "
"