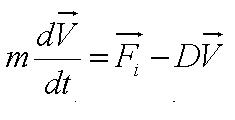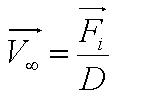Team:Imperial College/Motility
From 2008.igem.org
m |
m |
||
| Line 8: | Line 8: | ||
===== Materials ===== | ===== Materials ===== | ||
| - | [[Image:BSub- | + | [[Image:BSub-1.gif|frame|right|250px]] |
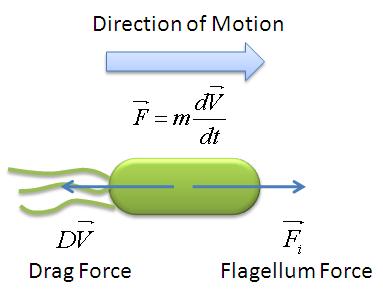
We used the Zeiss Axiovert 200 inverted microscope and Improvision Volocity acquisition software. This system offers a full incubation chamber with temperature control and a highly sensitive 1300x1000 pixel camera for fast low-light imaging. Video images are captured into memory by the system at a basal video frame rate of 16.3Hz. This can be further increased to 27.9Hz by performing x4 binning. | We used the Zeiss Axiovert 200 inverted microscope and Improvision Volocity acquisition software. This system offers a full incubation chamber with temperature control and a highly sensitive 1300x1000 pixel camera for fast low-light imaging. Video images are captured into memory by the system at a basal video frame rate of 16.3Hz. This can be further increased to 27.9Hz by performing x4 binning. | ||
A short video of swimming ''B. subtilis'' is shown: | A short video of swimming ''B. subtilis'' is shown: | ||
Revision as of 18:08, 29 October 2008
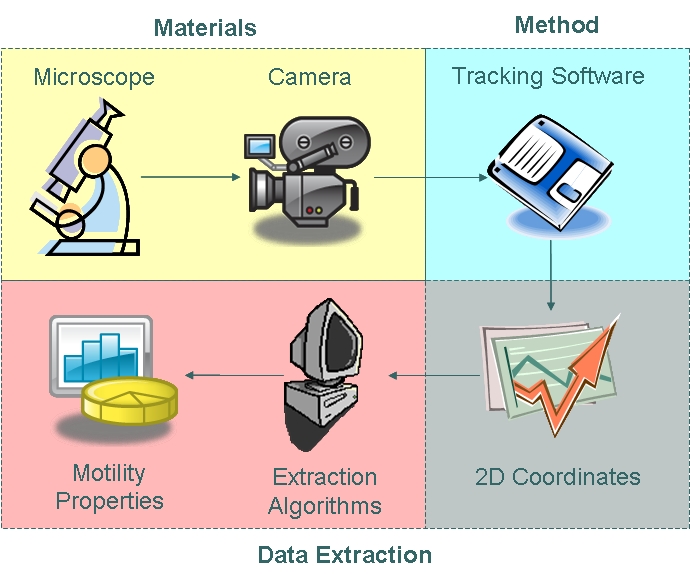
Motility Analysis
|
|||||||||||||||
 "
"