Team:Michigan/Project/Fabrication
From 2008.igem.org
(Difference between revisions)
| (14 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
<font color=navy> | <font color=navy> | ||
| - | = '''<font color= | + | = '''<font color=royalblue size=6>Project Fabrication</font>''' = |
| - | + | <font size=3>We will be using landing pads to insert the Sequestillator onto the chromosome of E. coli. Noisy behavior has proven detrimental to clock studies throughout the past, and we hope to reduce the noise in our system using landing pads.</font> | |
| - | <div align=center>[[Image: | + | |
| + | ==<font size=5 color=royalblue>Landing Pad Plans for Sequestillator</font>== | ||
| + | |||
| + | |||
| + | {|class="wikitable" border="0" cellpadding="10" cellspacing="1" style="padding: 1px; background-color:dodgerblue; border: 1px solid mediumblue; text-align:center" | ||
| + | !width="10%" align="justify" valign="top" style="background:gold; color:navy"| | ||
| + | |||
| + | ==<font color=royalblue size=3>Operon</font>== | ||
| + | <br><br><font size=3>Activator Operon</font><br><br><br> | ||
| + | |||
| + | == == | ||
| + | <br><br><font size=3>Repressor Operon</font> | ||
| + | |||
| + | !width="10%" align="justify" valign="top" style="background:gold; color:navy"| | ||
| + | |||
| + | ==<font color=royalblue size=3>Topology</font>== | ||
| + | |||
| + | <div align=center>[[Image:New 1st operon - gold.PNG|220px]]<br><br> | ||
| + | |||
| + | == == | ||
| + | <br>[[Image:New 2nd operon - gold.PNG|300px]]</div> | ||
| + | |||
| + | !width="10%" align="justify" valign="top" style="background:gold; color:navy"| | ||
| + | |||
| + | ==<font color=royalblue size=3>Landing Pad</font>== | ||
| + | |||
| + | <div align=left><font size=3>Leucine Landing Pad</font></div><br>constructed by former Ninfa lab member, Don Eun Chang | ||
| + | <br> | ||
| + | |||
| + | == == | ||
| + | <div align=left><font size=3>Arabinose Landing Pad</font></div><br> iGEM 2007 project by Alyssa Delke & Khalid Miri | ||
| + | |||
| + | |} | ||
| + | |||
| + | |||
| + | ==<font size=5 color=royalblue>Specific Fabrication Techniques</font>== | ||
| + | |||
| + | |||
| + | ==<font size=4 color=royalblue>Activator Operon</font>== | ||
| + | |||
| + | {| class="wikitable" border="0" cellpadding="10" cellspacing="1" style="padding: 5px; background-color:transparent; border: 1px solid transparent;text-align:center" | ||
| + | !width="70%" align="justify" valign="top" style="background:transparent; color:navy"| | ||
| + | |||
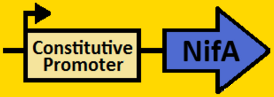
| + | <br><font size=3 color=royalblue>Constitutive Promoter</font> | ||
| + | *pArabLP was [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and NdeI | ||
| + | *BioBrick constitutive promoters were double [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] EcoRI and NdeI. | ||
| + | *Digests were [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol purified] using QIAGEN Purification Kit | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/LigationProtocol Ligation] was done overnight in 4⁰C. | ||
| + | *pArabLP-CP was then [https://2008.igem.org/Team:Michigan/Notebook/TransformationProtocol transformed] into E.coli competent cells and grown overnight on LB+Amp+Cm plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp+Cm overnight in 37⁰C. | ||
| + | *Plasmids from cells were [https://2008.igem.org/Team:Michigan/Notebook/PlasmidPrepProtocol isolated] by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and SpeI overnight in 37⁰C (to ensure proper ligation). | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/GelProtocol Analytical gel] was then run to check fragment size of approximately 50bp. | ||
| + | |||
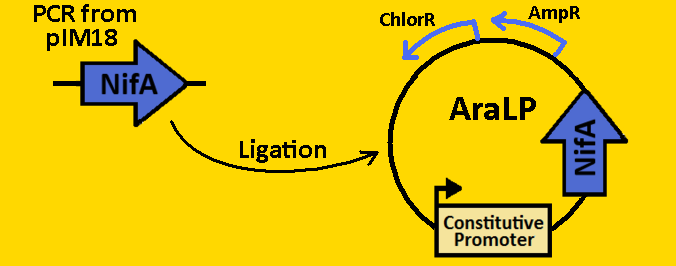
| + | <br><br><font size=3 color=royalblue>NifA</font> | ||
| + | *NifA was [https://2008.igem.org/Team:Michigan/Notebook/PCRProtocol amplified] from pRT22 using primers that had EcoRI, XbaI, NdeI and SpeI, PstI restriction sites flanking the 1560bp mutant sequence. | ||
| + | *The NifA sequence was then [https://2008.igem.org/Team:Michigan/Notebook/PCRProtocol purified] using the QIAGEN QIAquick PCR Purification Kit. | ||
| + | *NifA was double [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with NdeI and SpeI and left overnight in 37⁰C. | ||
| + | *pArabLP-CP was double [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with NdeI and SpeI also overnight at 37⁰C. | ||
| + | *Digests were [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol purified] using QIAGEN Purification Kit | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/LigationProtocol Ligation] was done overnight in 4⁰C. | ||
| + | *pArabLP-NifA was then [https://2008.igem.org/Team:Michigan/Notebook/TransformationProtocol transformed] into E.coli competent cells and grown overnight on LB+Amp+Cm plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp+Cm overnight in 37⁰C. | ||
| + | *Plasmids from cells were [https://2008.igem.org/Team:Michigan/Notebook/PlasmidPrepProtocol isolated] by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and SpeI overnight in 37⁰C (to ensure proper ligation). | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/GelProtocol Analytical gel] was then run to check fragment size of approximately 1650bp. | ||
| + | |||
| + | |||
| + | !width="10%" align="justify" valign="top" style="background:transparent; color:navy"| | ||
| + | |||
| + | <br><br><br>[[Image:Fabrication - constitutive promoter.PNG|400px]] | ||
| + | <br><br><br><br><br><br><br><br><br>[[Image:Fabrication - nifA.PNG|400px]] | ||
| + | |||
| + | |} | ||
| + | |||
| + | ==<font size=4 color=royalblue>Repressor Operon</font>== | ||
| + | |||
| + | {| class="wikitable" border="0" cellpadding="10" cellspacing="1" style="padding: 5px; background-color:transparent; border: 1px solid transparent;text-align:center" | ||
| + | !width="70%" align="justify" valign="top" style="background:transparent; color:navy"| | ||
| + | |||
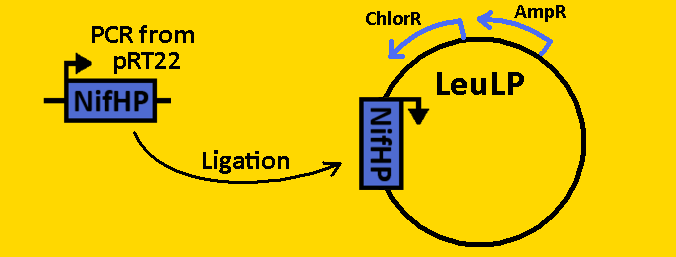
| + | <font size=3 color=royalblue>NifHp</font> | ||
| + | *The NifH promoter (NifHp) was [https://2008.igem.org/Team:Michigan/Notebook/PCRProtocol amplified] from pRT22 using primers that had EcoRI, XbaI and NdeI, SpeI, PstI restriction sites flanking the 290bp sequence. | ||
| + | *The NifHp sequence was then [https://2008.igem.org/Team:Michigan/Notebook/PCRProtocol purified] using the QIAGEN QIAquick PCR Purification Kit. | ||
| + | *NifHp was double [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and SpeI and left overnight in 37⁰C. | ||
| + | *pLeuLP was double [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and SpeI also overnight at 37⁰C. | ||
| + | *Digests were [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol purified] using QIAGEN Purification Kit | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/LigationProtocol Ligation] was done overnight in 4⁰C. Newly formed plasmid is renamed to pCLOCK1 | ||
| + | *pCLOCK1 was then [https://2008.igem.org/Team:Michigan/Notebook/TransformationProtocol transformed] into E.coli competent cells and grown overnight on LB+Amp+Cm plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp+Cm overnight in 37⁰C. | ||
| + | *Plasmids from cells were [https://2008.igem.org/Team:Michigan/Notebook/PlasmidPrepProtocol isolated] by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and SpeI overnight in 37⁰C (to ensure proper ligation). | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/GelProtocol Analytical gel] was then run to check fragment size of approximately 300bp. | ||
| + | |||
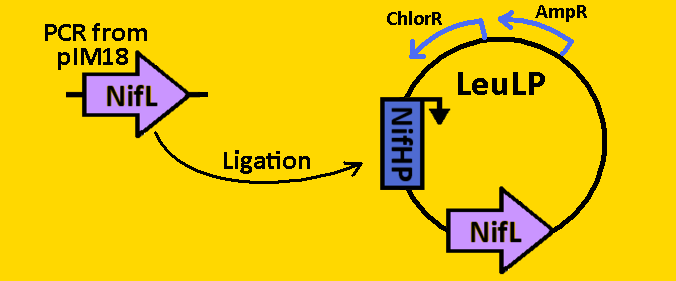
| + | <br><br><font size=3 color=royalblue>NifL</font> | ||
| + | *NifL was [https://2008.igem.org/Team:Michigan/Notebook/PCRProtocol amplified] from pIM18 using primers that had EcoRI, XbaI, NdeI and SpeI, PstI restriction sites flanking the 1590bp mutant sequence. | ||
| + | *The NifL sequence was then [https://2008.igem.org/Team:Michigan/Notebook/PCRProtocol purified] using the QIAGEN QIAquick PCR Purification Kit. | ||
| + | *NifL was double [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with NdeI and SpeI and left overnight in 37⁰C. | ||
| + | *pLeuLP with NifHP was double [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with NdeI and SpeI also overnight at 37⁰C. | ||
| + | *Digests were [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol purified] using QIAGEN Purification Kit | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/LigationProtocol Ligation] was done overnight in 4⁰C. Newly formed plasmid is renamed to pCLOCK2 | ||
| + | *pCLOCK2 was then [https://2008.igem.org/Team:Michigan/Notebook/TransformationProtocol transformed] into E.coli competent cells and grown overnight on LB+Amp+Cm plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp+Cm overnight in 37⁰C. | ||
| + | *Plasmids from cells were [https://2008.igem.org/Team:Michigan/Notebook/PlasmidPrepProtocol isolated] by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and SpeI overnight in 37⁰C (to ensure proper ligation). | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/GelProtocol Analytical gel] was then run to check fragment size of approximately 2000bp. | ||
| + | |||
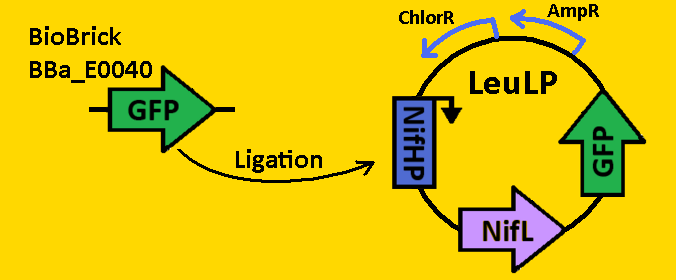
| + | <br><br><font size=3 color=royalblue>GFP </font> | ||
| + | *pClock2 was [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and SpeI | ||
| + | *BioBrick BBa_E0040 that contained GFP was [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and XbaI in sequential digests. | ||
| + | *Digests were [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol purified] using QIAGEN Purification Kit | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/LigationProtocol Ligation] into pLeuLP with NifHP and NifL was done overnight in 4⁰C. | ||
| + | *pCLOCK2 was then [https://2008.igem.org/Team:Michigan/Notebook/TransformationProtocol transformed] into E.coli competent cells and grown overnight on LB+Amp plates in 37⁰C. | ||
| + | *Single colonies were isolated from the plates and grown in 5mL liquid culture containing LB+Amp overnight in 37⁰C. | ||
| + | *Plasmids from cells were [https://2008.igem.org/Team:Michigan/Notebook/PlasmidPrepProtocol isolated] by using QIAGEN MiniPrep Kit | ||
| + | *Plasmids were then [https://2008.igem.org/Team:Michigan/Notebook/DigestProtocol digested] with EcoRI and SpeI overnight in 37⁰C (to ensure proper ligation). | ||
| + | *[https://2008.igem.org/Team:Michigan/Notebook/GelProtocol Analytical gel] was then run to check fragment size of approximately 2700bp. | ||
| + | |||
| + | !width="10%" align="justify" valign="top" style="background:transparent; color:navy"| | ||
| + | |||
| + | <br><br><br><br>[[Image:Fabrication - nifHP.PNG|400px]] | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br>[[Image:Fabrication - nifL.PNG|400px]] | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br>[[Image:Fabrication - GFP.PNG|400px]] | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | <div align=center>For better visualization of what our parts look like in our landing pads, see our [[Team:Michigan/Project/LandingPads | Landing Pad Page]]</div> | ||
|} | |} | ||
Latest revision as of 03:53, 30 October 2008
|
|---|
|
Project FabricationWe will be using landing pads to insert the Sequestillator onto the chromosome of E. coli. Noisy behavior has proven detrimental to clock studies throughout the past, and we hope to reduce the noise in our system using landing pads.
Landing Pad Plans for Sequestillator
Specific Fabrication TechniquesActivator Operon
Repressor Operon
For better visualization of what our parts look like in our landing pads, see our Landing Pad Page
|
|---|
 "
"