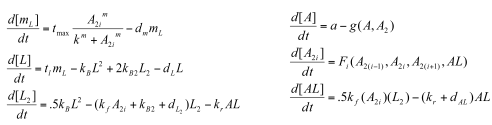Team:Michigan/Project/Modeling/Model2
From 2008.igem.org
(New page: __NOTOC__ {{Michigan Header}} {|class="wikitable" border="0" cellpadding="10" cellspacing="1" style="padding: 1px; background-color:dodgerblue; border: 1px solid mediumblue; text-align:...) |
|||
| Line 28: | Line 28: | ||
*A= NifA protein | *A= NifA protein | ||
*A2i= NifA complex, where i=1 is a dimer, i=4 is a tetramer, and i=6 is a hexamer | *A2i= NifA complex, where i=1 is a dimer, i=4 is a tetramer, and i=6 is a hexamer | ||
| - | + | Note, we condensed the mRNA NifA reactions to be encapsulated in the 'a' production term. <br> | |
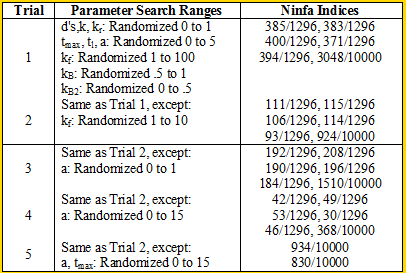
We ran some Ninfa Index simulations on the i=1 model (the other models were too complex to run the simulations on [my MacBook lacks computing power]): | We ran some Ninfa Index simulations on the i=1 model (the other models were too complex to run the simulations on [my MacBook lacks computing power]): | ||
<div align='center'> [[Image: Table2.png]]</div> | <div align='center'> [[Image: Table2.png]]</div> | ||
| Line 34: | Line 34: | ||
<br> When we decrease the randomization range of kf to in between 0 and 10 for trial 2, we see an almost threefold to fourfold decrease in the Ninfa index. Comparing trials 2 and 3 to Trial 1, we see that if we disturb the "equilibrium" of the system by producing lots of NifA (in trial 3) or too little Nifa (in trial 1), we can lose a lot of oscillatory potential. We seen increase in the Ninfa index when we "balance" the system by increasing range of the maximal transcription rate of NifL, as seen in Trial 4. | <br> When we decrease the randomization range of kf to in between 0 and 10 for trial 2, we see an almost threefold to fourfold decrease in the Ninfa index. Comparing trials 2 and 3 to Trial 1, we see that if we disturb the "equilibrium" of the system by producing lots of NifA (in trial 3) or too little Nifa (in trial 1), we can lose a lot of oscillatory potential. We seen increase in the Ninfa index when we "balance" the system by increasing range of the maximal transcription rate of NifL, as seen in Trial 4. | ||
| + | FURTHER WORK THAT WILL BE PRESENTED DURING iGEM POSTER TALKS | ||
| + | *Cloud pictures of these results | ||
| + | *A more in depth model that accounts for NifA mRNA production. | ||
[https://2008.igem.org/Team:Michigan/Project/Modeling Back to Modeling] | [https://2008.igem.org/Team:Michigan/Project/Modeling Back to Modeling] | ||
|} | |} | ||
Latest revision as of 03:07, 30 October 2008
|
|---|
|
Sequestillator Model 2: A More Complicated ModelWhile Model 1 gave us some important results (mainly: need a low Kd for oscillations to occur) , we decided to look at more complete model that accounted for some of the dimerizations the proteins undergo: Parameters
Functions
Variables
Note, we condensed the mRNA NifA reactions to be encapsulated in the 'a' production term. Overall, we see smaller indices than the ones we saw in Model 1. Nonetheless,we see that the clock has at least some chance of oscillating.
FURTHER WORK THAT WILL BE PRESENTED DURING iGEM POSTER TALKS
|
|---|
 "
"