Team:NYMU-Taipei/Project/Attachment/FimH
From 2008.igem.org
(Difference between revisions)
Blackrabbit (Talk | contribs)
(New page: ====FimH Binding Domain Structure==== *FimH can bind to different kind of mannose on human bladder or intestine epitheial cells. *FimH has different variants. Monomannose-specific phenotyp...)
Newer edit →
(New page: ====FimH Binding Domain Structure==== *FimH can bind to different kind of mannose on human bladder or intestine epitheial cells. *FimH has different variants. Monomannose-specific phenotyp...)
Newer edit →
Revision as of 17:46, 29 October 2008
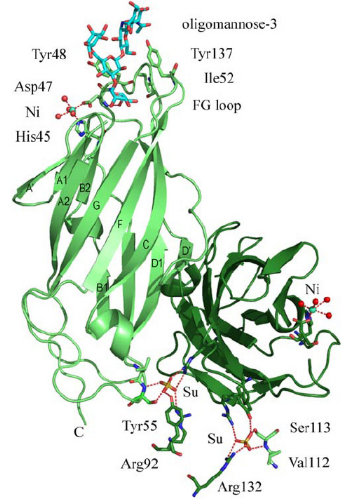
FimH Binding Domain Structure
- FimH can bind to different kind of mannose on human bladder or intestine epitheial cells.
- FimH has different variants. Monomannose-specific phenotype is dominant among uropathogenic E. coli while the oligomannose phenotype is most common among fecal E. coli.
- heptyl-a-D-mannose prevents binding of type 1-piliated E. coli to the human bladder cell line 5637.
- As the figure show, FimH has two domains:
- the upper domain is a lectine domain--- the mannose binding site.
- the other is a pilin domain---controlling the affinity between FimH and mannose according to different shear strain .
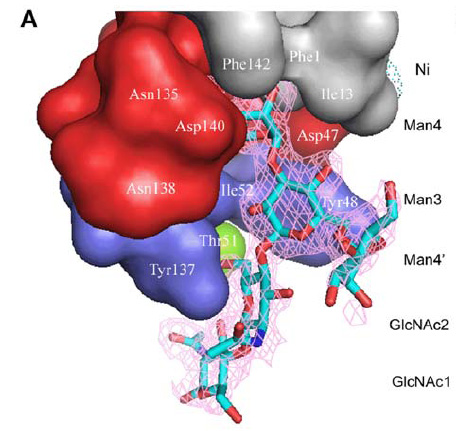
- the binding domain is a kind of lectin.It can be devided in three parts:
- polar pocket(red part):binds to mannose with hydrogen bond.
- tyrosine gate(blue part):binds to mannose with aromatic stacking,van der waals force and hydrophobic contact.
- hydrophobic support platform(grey part)
- The mannose shows in the figure is Oligomannose-3.
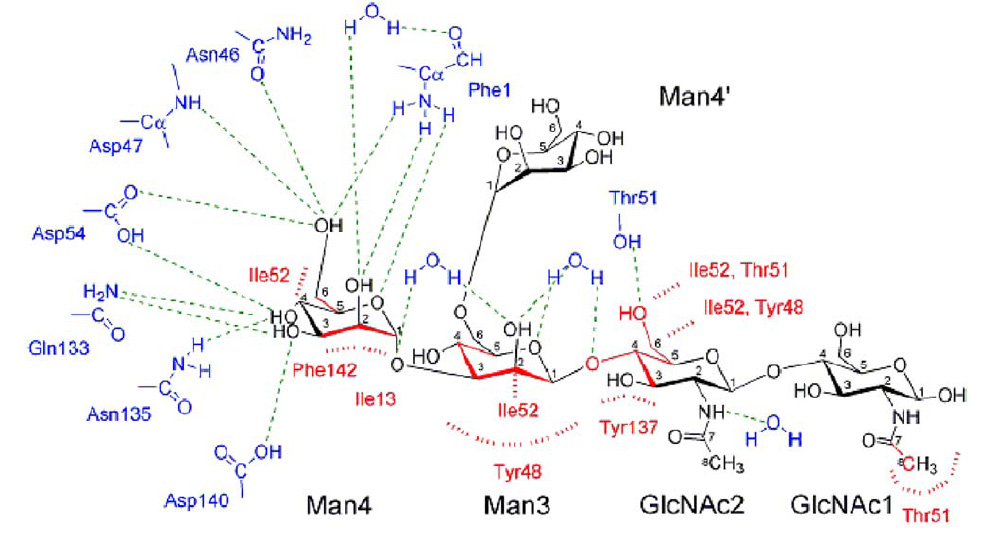
FimH Lectin Binding Domain & mannose interaction
- The blue and red parts are FimH amino acid, while black part is oligomannose.
- cyan dashes mean hydrogen bond, and red dashes mean aromatic stacking or van der waals force.
- from the picture, we can see that Man4 interacts with FimH through polar pocket with hydrogen bond, while Man3,GLcNAc2,GLcNAc1 interact with tyrosine gate by aromatic stacking & van der waals force.
 "
"