Team:Newcastle University/Conclusions
From 2008.igem.org
Riachalder (Talk | contribs) |
Riachalder (Talk | contribs) |
||
| Line 5: | Line 5: | ||
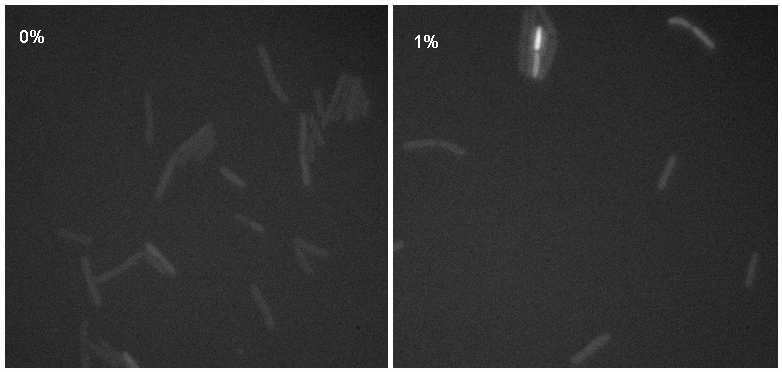
[[Image:Montage.jpg|thumb|right|300px|Microscopy results of iGEMgfp upon 1% induction by subtilin]] | [[Image:Montage.jpg|thumb|right|300px|Microscopy results of iGEMgfp upon 1% induction by subtilin]] | ||
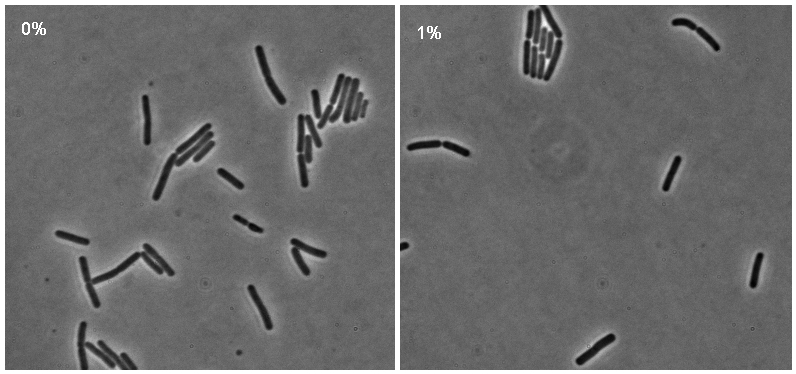
[[Image:Montage_pc.jpg|thumb|right|300px|Microscopy results of iGEMcherry upon 1% induction by subtilin]] | [[Image:Montage_pc.jpg|thumb|right|300px|Microscopy results of iGEMcherry upon 1% induction by subtilin]] | ||
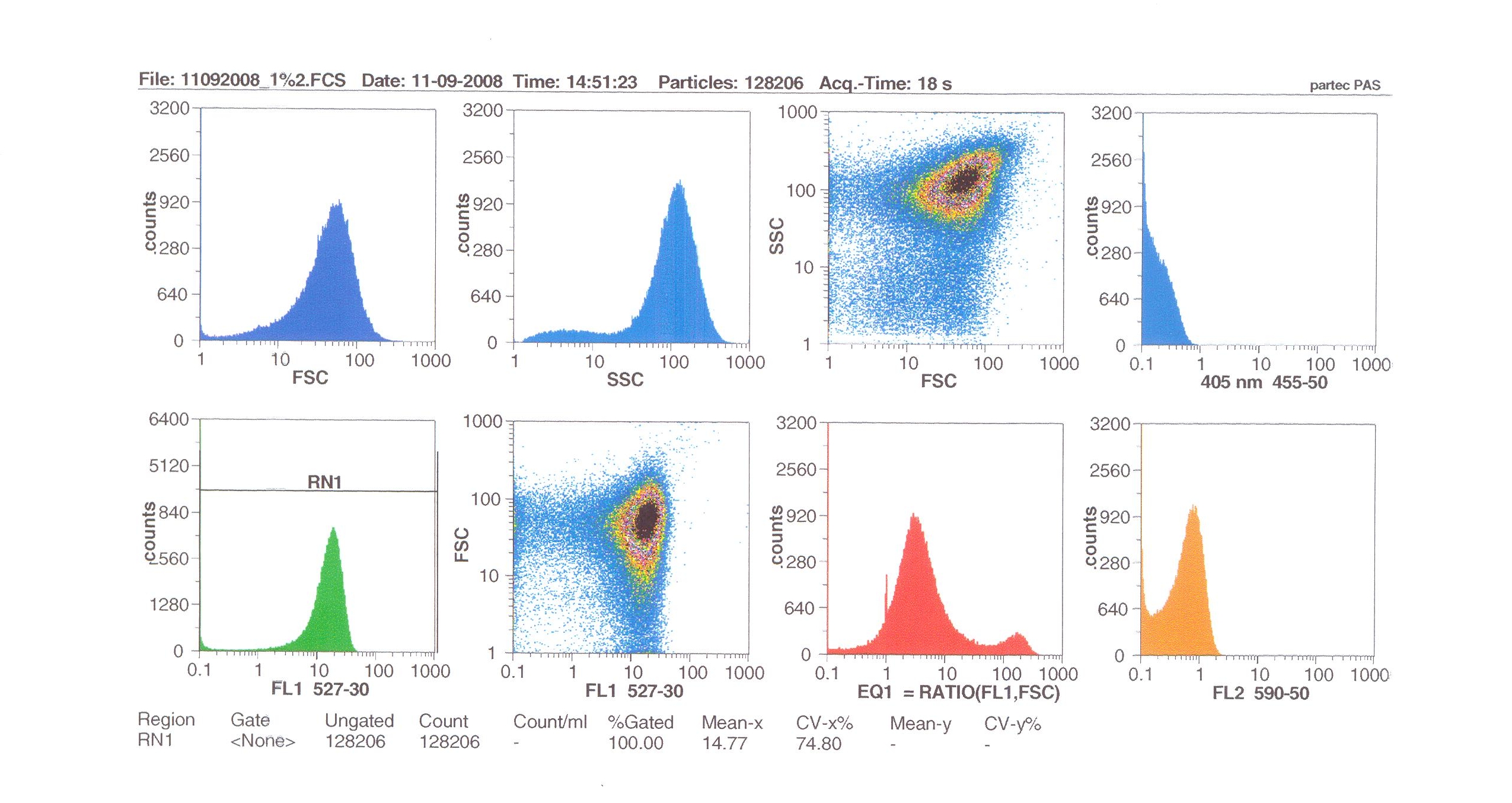
| - | + | [[Image:Flow1%.jpg|thumb|right|300px|Flow cytometry results for 1% induction by subtilin.]] | |
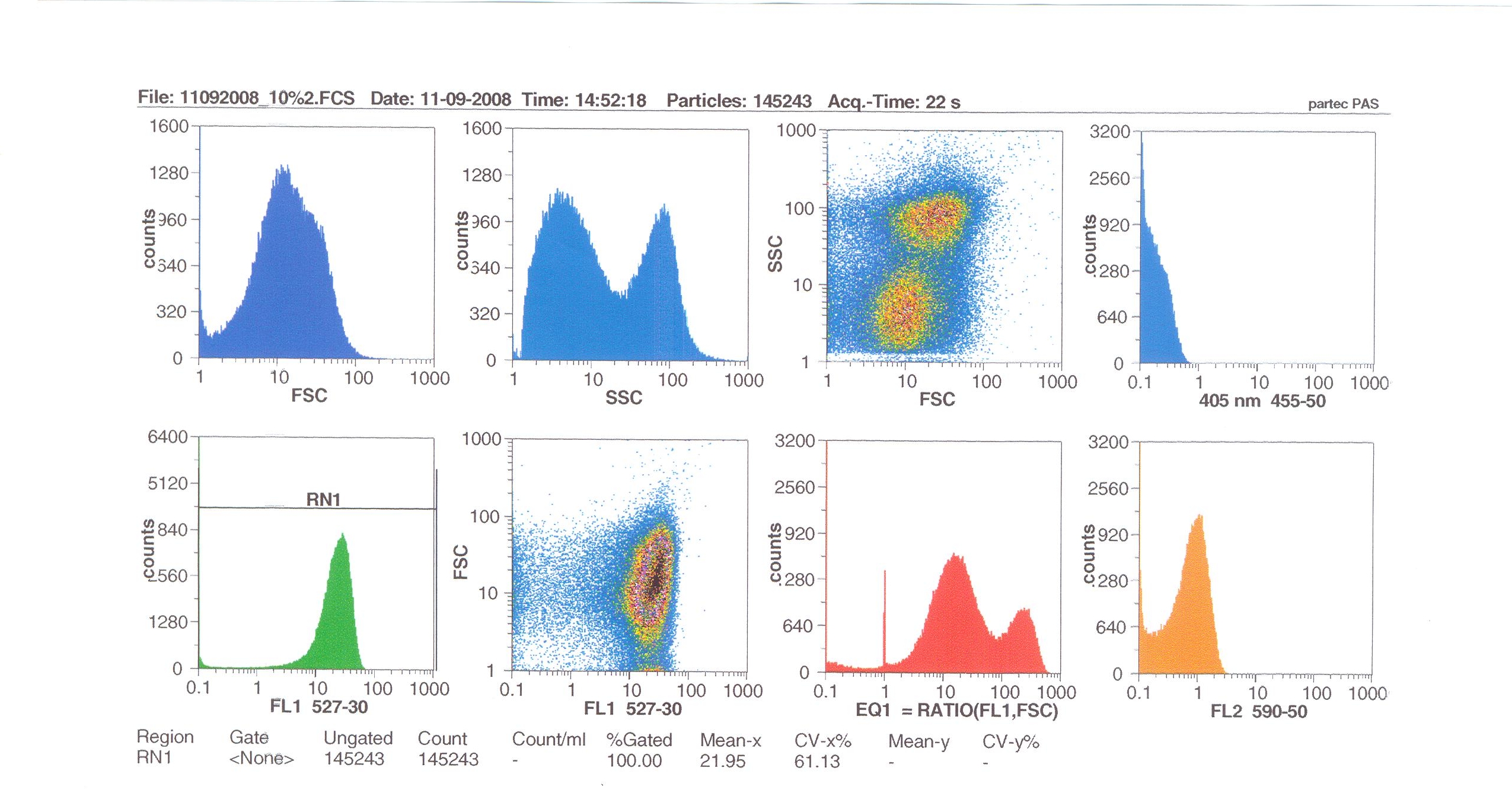
| + | [[Image:Flow10%.jpg|thumb|right|300px|Flow cytometry results for 10% induction by subtilin.]] | ||
<html> | <html> | ||
</div><!--maincontent --> | </div><!--maincontent --> | ||
</html> | </html> | ||
| - | To analyse the results of the wet lab transformations of the inserts into ''B. subtilis'', we used two methods: | + | To analyse the results of the wet lab transformations of the inserts into ''B. subtilis'', we used two methods: microscopy and flow cytometry. |
| + | |||
| + | Microscopy work done on 08.09.08 showed a possible difference in the brightness of the iGEMgfp fluorescent cells (brighter in 10% subtilin-induced cells than in 0% subtilin-induced cells). However, there was little difference in the numbers of cells that fluoresced between the two cultures. There was no difference in either the number of fluorecent cells or their brightness between the 10% subtilin-induced and the 0% subtilin-induced iGEMcherry cells. | ||
| + | |||
| + | ===Flow cytometry=== | ||
| - | + | Flow cytometry allows us to quantify our results and present them in graphical form. A sample of cells our engineered Bacillus subtilis cells were injected into the machine which hydro-dynamically focusses the fluid. Lasers are directed onto the stream of fluid, and each particle which passes through the light beam will cause the laser to scatter in a particular way. Fluorescent chemicals will be excited and emit light at a higher wavelength than the light source. | |
| - | + | The detectors in the machine measure the scattering of light and any flourescence which occurs. | |
| - | |||
<div id="sidebar"> | <div id="sidebar"> | ||
Revision as of 13:12, 19 September 2008
Newcastle University
GOLD MEDAL WINNER 2008
| Home | Team | Original Aims | Software | Modelling | Proof of Concept Brick | Wet Lab | Conclusions |
|---|
Results
To analyse the results of the wet lab transformations of the inserts into B. subtilis, we used two methods: microscopy and flow cytometry.
Microscopy work done on 08.09.08 showed a possible difference in the brightness of the iGEMgfp fluorescent cells (brighter in 10% subtilin-induced cells than in 0% subtilin-induced cells). However, there was little difference in the numbers of cells that fluoresced between the two cultures. There was no difference in either the number of fluorecent cells or their brightness between the 10% subtilin-induced and the 0% subtilin-induced iGEMcherry cells.
Flow cytometry
Flow cytometry allows us to quantify our results and present them in graphical form. A sample of cells our engineered Bacillus subtilis cells were injected into the machine which hydro-dynamically focusses the fluid. Lasers are directed onto the stream of fluid, and each particle which passes through the light beam will cause the laser to scatter in a particular way. Fluorescent chemicals will be excited and emit light at a higher wavelength than the light source.
The detectors in the machine measure the scattering of light and any flourescence which occurs.
 "
"