Team:Paris/July 30
From 2008.igem.org
(→Results of transformations) |
|||
| Line 220: | Line 220: | ||
|align="center"|Amp | |align="center"|Amp | ||
|align="center"|a lot | |align="center"|a lot | ||
| - | |align="center"| | + | |align="center"| |
|- | |- | ||
|align="center"|L125 | |align="center"|L125 | ||
| Line 226: | Line 226: | ||
Strongest RBS - yfp | Strongest RBS - yfp | ||
<br>D102 (BV) - D118 (BI) | <br>D102 (BV) - D118 (BI) | ||
| + | |align="center"|Amp | ||
| + | |align="center"|a lot | ||
|align="center"| | |align="center"| | ||
| - | |||
| - | |||
| - | |||
|- | |- | ||
|align="center"|L126 | |align="center"|L126 | ||
| Line 235: | Line 234: | ||
Strongest RBS (1)- LacR activator (+LVA) | Strongest RBS (1)- LacR activator (+LVA) | ||
<br>D102 (BV) - D118 (BI) | <br>D102 (BV) - D118 (BI) | ||
| - | |align="center"| | + | |align="center"|Amp |
|align="center"|no dish found!!! | |align="center"|no dish found!!! | ||
| - | |||
|align="center"|To do again | |align="center"|To do again | ||
|- | |- | ||
| Line 244: | Line 242: | ||
gfp (1)- Double terminator | gfp (1)- Double terminator | ||
<br>D119 (FI) - D125 (FV) | <br>D119 (FI) - D125 (FV) | ||
| - | |align="center"| | + | |align="center"|Amp |
|align="center"|no dish found!!! | |align="center"|no dish found!!! | ||
| - | |||
|align="center"|To do again | |align="center"|To do again | ||
|- | |- | ||
|align="center"|'''Controls''' | |align="center"|'''Controls''' | ||
| - | |||
|align="center"| | |align="center"| | ||
|align="center"| | |align="center"| | ||
| Line 261: | Line 257: | ||
|align="center"| | |align="center"| | ||
|align="center"|0 | |align="center"|0 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 269: | Line 264: | ||
|align="center"| | |align="center"| | ||
|align="center"|5 | |align="center"|5 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 277: | Line 271: | ||
|align="center"| | |align="center"| | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 285: | Line 278: | ||
|align="center"| | |align="center"| | ||
|align="center"|+/- 20 | |align="center"|+/- 20 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 293: | Line 285: | ||
|align="center"| | |align="center"| | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 301: | Line 292: | ||
|align="center"| | |align="center"| | ||
|align="center"|+/- 100 | |align="center"|+/- 100 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 309: | Line 299: | ||
|align="center"| | |align="center"| | ||
|align="center"|0 | |align="center"|0 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 317: | Line 306: | ||
|align="center"| | |align="center"| | ||
|align="center"|+/- 20 | |align="center"|+/- 20 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 325: | Line 313: | ||
|align="center"| | |align="center"| | ||
|align="center"|0 | |align="center"|0 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 333: | Line 320: | ||
|align="center"| | |align="center"| | ||
|align="center"|0 | |align="center"|0 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 341: | Line 327: | ||
|align="center"| | |align="center"| | ||
|align="center"|0 | |align="center"|0 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 349: | Line 334: | ||
|align="center"| | |align="center"| | ||
|align="center"|+/- 10 | |align="center"|+/- 10 | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|- | |- | ||
| Line 357: | Line 341: | ||
|align="center"| | |align="center"| | ||
|align="center"|155 (transformation efficiency:1.5*10^7/ug) | |align="center"|155 (transformation efficiency:1.5*10^7/ug) | ||
| - | |||
|align="center"|- | |align="center"|- | ||
|} | |} | ||
| - | |||
==Analysis of yesterday DNA digestion== | ==Analysis of yesterday DNA digestion== | ||
Revision as of 09:27, 5 August 2008
|
Results of transformations
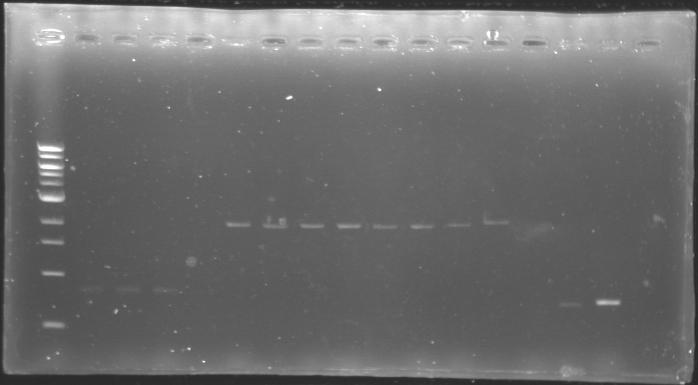
Analysis of yesterday DNA digestionThe digested DNA of yesterday was analysed one more time by electrophoresis on a 0.8% agarose gel (about 30 minutes at 100 W). The ladder used was the 1 kb DNA ladder (New England Biolabs). 5 µL of each sample with 1 µL of loading dye were loaded.
Results : Each of the samples was succesfully digested and purified except for the sample D108. It seems that the QIAprep columms (from the QIAGEN Minipreps kit) can be used instead of the QIAquick columms (for DNA Gel Extraction). |
 "
"