Team:Paris/Perspectives
From 2008.igem.org
Philippe b (Talk | contribs) (→A.DNA nanocar factory) |
Philippe b (Talk | contribs) (→B.Artificial virus factory) |
||
| Line 50: | Line 50: | ||
* Finaly, when 23 are activated alone, we will increase the quantity of subunits delivered from the clivage of Env. | * Finaly, when 23 are activated alone, we will increase the quantity of subunits delivered from the clivage of Env. | ||
| - | |||
| - | |||
| - | |||
== Metabolic engineering of polyhydroxyalkanoate biosynthesis pathways == | == Metabolic engineering of polyhydroxyalkanoate biosynthesis pathways == | ||
Revision as of 15:04, 28 October 2008
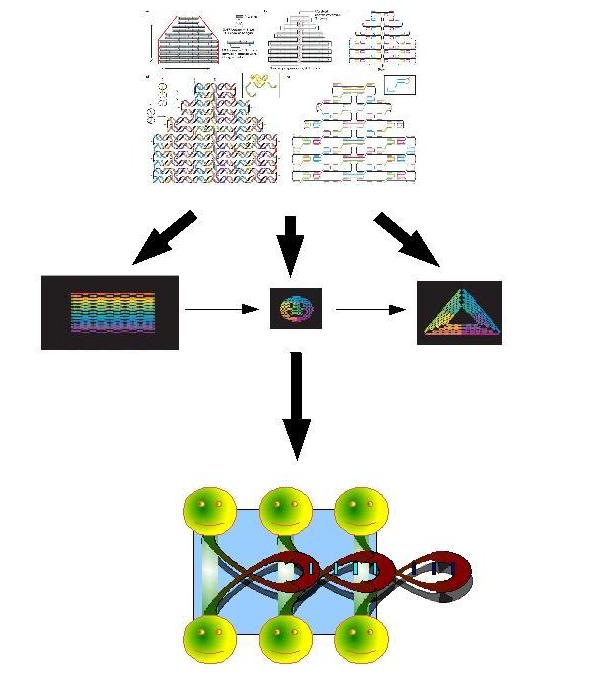
Biosynthetic Bottum-up approachA.DNA nanocar factoryThe field of artificial molecular machines and motors is growing at an astonishing rate and is attracting a great deal of interest in nanoscience. Research in the last decade has shown that species made of components like DNA or proteins are attractive candidates. Our aims is to build by a bottom-up approched DNA nanocars that have the properties to produce energy in vitro. You have to explain what you mean by "produce energy in vitro". What energy does it use? to convert into what other energy? or to use in what way? Also, why in vitro? Do you plan to use our system in cell-free extracts or in living cells? Our system is more efficient than a classic system because the FIFO order is very important: the first part will bind by complementarity with part 2 this new part will make the binding site for part 3. Fig.1:biosynthesis by sequential expression of 3 DNA origami If we add in vitro a complementary miRNA to the red regions, the competition of the miRNA will open the DNA strands by broking the hydrogen backbound this processes deliveres minimun ten time more energy than ATP biodegradation (winfree et.al), the instability of the miRNA may make this processe reversible. What is this red region? How will it allow to use the delivered energy? Plus it's not enough to cite a paper to prove that energy is delivered. If the miRNA is complementary to a region that is already paired, the energy you need to unpair this region is the same that you get when you pair it with the miRNA. Result: 0 energy gain.
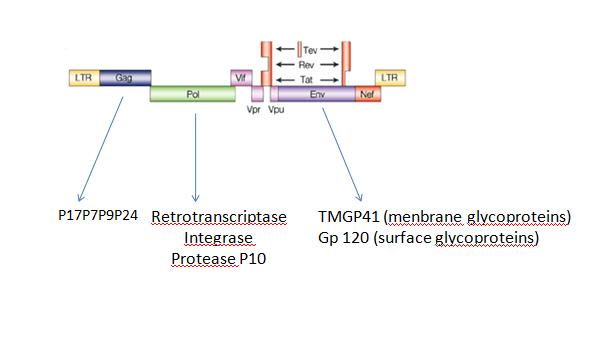
B.Artificial virus factoryWe can also produce a lot of differentes kinds of self-assembly structures like virus for example,HIV:
Fig2: genetic organization of HIV virus HIV can be produce by sequential expression of 3 genes gag,env and pol (Fig.2)those genes will be cleaved by protease P10 and than the subunits of the virus will self-assembled into mature virus. In our case, we will expressed gag p10 and finaly Env (without Pol to avoid pathogenicity) the FIFO in this case will be essential. If you ask us why? For 3 main reasons :
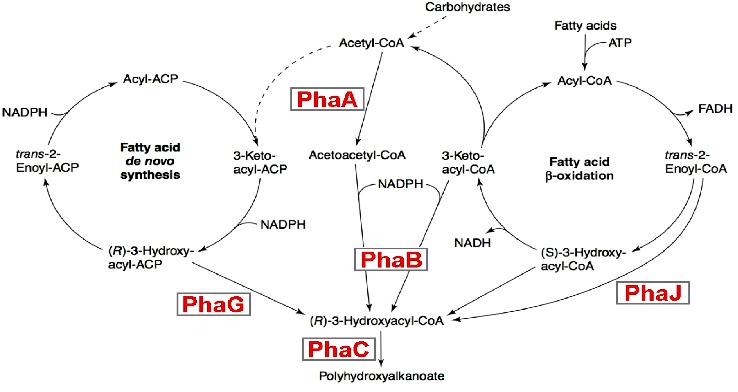
Metabolic engineering of polyhydroxyalkanoate biosynthesis pathwaysHuman overpopulation combined with the current lifestyle urges the rational, efficient, and sustainable use of natural resources to produce environmentally friendly plastic materials such as polyhydroxyalkanoic acids (PHAs), whose production/degradation cycle reduces undesirable wastes and emissions. Our study is a new metabolic strategy to generate PHA-hyperproducer strains, that have the properties to be a sequential metabolic pathway, we believe the sequential expression may increases the production of purified PHA. Our strategy consists on replacing the RFP,CFP and YFP genes by the PhaA ,PhaB and PhaC genes in our final system (containing oscillation,FIFO,synchronisation modules). This strategy is more efficient than a constitutive activation for 3 main reasons:
I'm not sure turnover has this meaning
Again, you cannot just state this boldly without constructing an argument. You never explain why your system is better than simply expressing all the genes continuously at a lower level More generally, on this whole page: We don't expect you to give very detailed projects. You just need to give simple ideas for which the interest of the FIFO seems obvious (or at least you need to try making it seem obvious). We are not so much interested in the precise mechanisms or genes involved, but rather in the principles that make your projects interesting. Bibliography
Paul W. K. Rothemund
Paul W. K. Rothemund
Suvir Venkataraman, Robert M. Dirks, Paul W. K. Rothemund, Erik Winfree, Niles A. Pierce.
Georg Seelig, Bernard Yurke, Erik Winfree. |
 "
"