Team:Paris/Quorum sensing
From 2008.igem.org
(→The genetic cascade) |
(→The genetic cascade) |
||
| Line 9: | Line 9: | ||
The AHL freely diffuses into the extracellular medium. Its concentration increases as the population gets more dense. When the concentration reaches a critical level, those small molecules bind to their receptor. LuxR bound to AHL and the complex activate the operon that code for luciferase (''Figure 1.''). | The AHL freely diffuses into the extracellular medium. Its concentration increases as the population gets more dense. When the concentration reaches a critical level, those small molecules bind to their receptor. LuxR bound to AHL and the complex activate the operon that code for luciferase (''Figure 1.''). | ||
| + | |||
| + | ===Quorum Sensing and Synthetic biology=== | ||
| + | This system has already been used in the field of synthetic biology to create new behaviors. For instance, [http://www.nature.com/nature/journal/v428/n6985/abs/nature02491.html Lingchong YOU ''et al.'' (2004)] designed a bacteria that regulates the maximum density of bacteria inducing a controlled suicide via intercellular communication. With this system, even if the population is stable the genome is expressed as if they were in exponential growth. | ||
| + | |||
| + | Other quorum sensing systems has been described in different species of baceteria such as ''Pseudomonas aeruginosa''. The system has the same architecture with protein LasI that synthesize the AHL that binds to LasR, the intracellular receptor. One of the differences between those to systems is the structure of the AHL (Figure 2.) | ||
===Bibliography=== | ===Bibliography=== | ||
Revision as of 18:03, 29 October 2008
Contents |
Presentation of the phenomenon of quorum-sensing : intercellular communication in bacterias
The phenomenon of quorum sensing was first discovered in Vibrio fischeri. This bacteria only expresses luciferase when the density of bacteria is very high (1011bact/mL). Typically, the bacteria does not express bioluminescence when they are free living in the ocean. At the contrary, they express luciferase when they are highly concentrated in the photophore of Hawaiian bobtail squid.
The genetic cascade
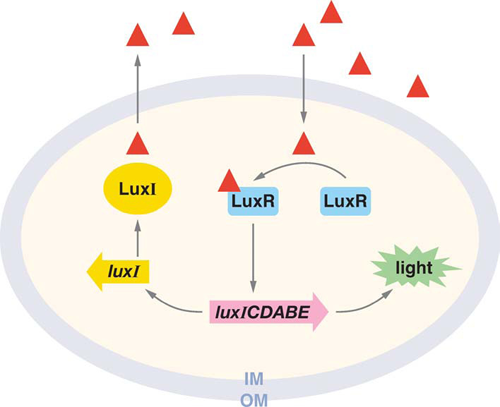
In this system, LuxI and LuxR control the expression of luciferase operon luxICDABE, necessary to produce bioluminescence. LuxI synthesize small diffusible molecules called Acyl Homoserin Lactone (AHL). LuxR is the intracellular receptor to AHL.
The AHL freely diffuses into the extracellular medium. Its concentration increases as the population gets more dense. When the concentration reaches a critical level, those small molecules bind to their receptor. LuxR bound to AHL and the complex activate the operon that code for luciferase (Figure 1.).
Quorum Sensing and Synthetic biology
This system has already been used in the field of synthetic biology to create new behaviors. For instance, Lingchong YOU et al. (2004) designed a bacteria that regulates the maximum density of bacteria inducing a controlled suicide via intercellular communication. With this system, even if the population is stable the genome is expressed as if they were in exponential growth.
Other quorum sensing systems has been described in different species of baceteria such as Pseudomonas aeruginosa. The system has the same architecture with protein LasI that synthesize the AHL that binds to LasR, the intracellular receptor. One of the differences between those to systems is the structure of the AHL (Figure 2.)
Bibliography
x Christopher M Waters et Bonnie L Bassler, “Quorum sensing: cell-to-cell communication in bacteria,” Annual Review of Cell and Developmental Biology 21 (2005): 319-46, doi:10.1146/annurev.cellbio.21.012704.131001. xi A M Stevens, K M Dolan, et E P Greenberg, “Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region,” Proceedings of the National Academy of Sciences of the United States of America 91, no. 26 (Décembre 20, 1994): 12619-23, doi:7809088. xii Lingchong You et coll., “Programmed population control by cell-cell communication and regulated killing,” Nature 428, no. 6985 (Avril 22, 2004): 868-871, doi:10.1038/nature02491. xiii Frederick K Balagaddé et coll., “A synthetic Escherichia coli predator-prey ecosystem,” Molecular Systems Biology 4 (2008): 187, doi:msb200824
 "
"