Team:Paris/September 8
From 2008.igem.org
(Difference between revisions)
(→Digestion) |
(→Purification) |
||
| Line 62: | Line 62: | ||
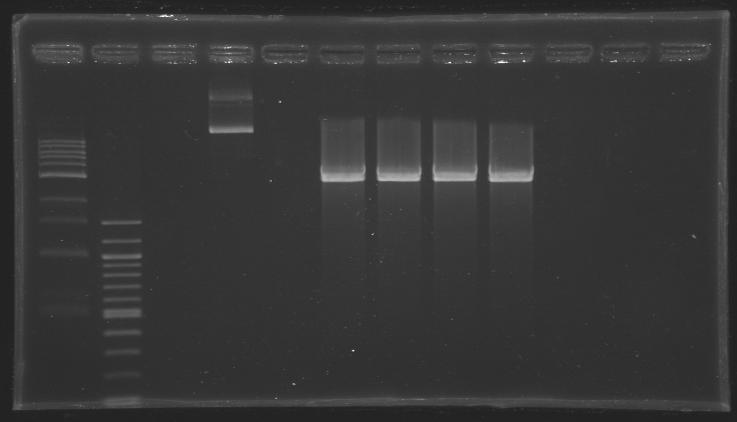
*We purifed our digestion products by gel extraction (Qiagen kit) | *We purifed our digestion products by gel extraction (Qiagen kit) | ||
| - | *After elution, we obtained [DNA] | + | *After elution, we obtained [DNA] ~ 25 ng/µL (based on the intensity of the band) |
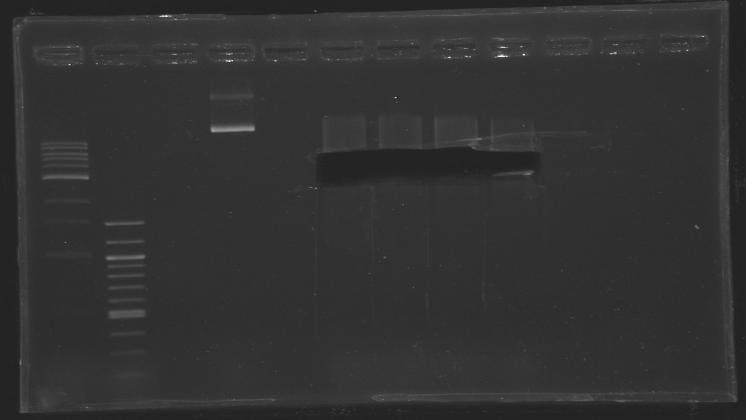
== Overnight ligation (16°C)== | == Overnight ligation (16°C)== | ||
Revision as of 14:30, 12 September 2008
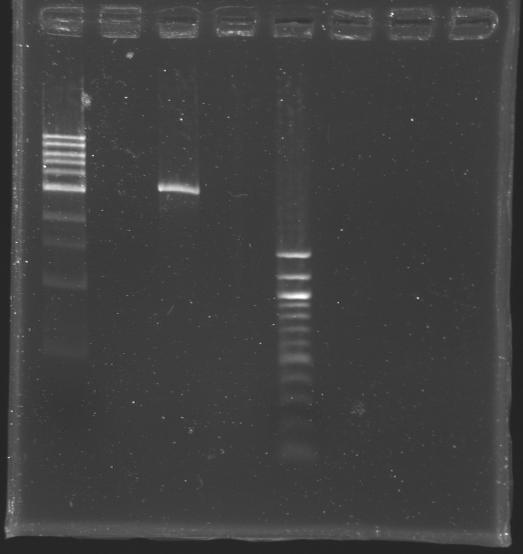
Checking our ligasesSince our ligation experiments didn't work during these few days, we presume that our problem came from the ligases. That's why we decided to check the activity of the ligases in our possession. To do so:
Condition tested
DigestionReaction mixture (carried out four times )
Purification
Overnight ligation (16°C)
|
 "
"