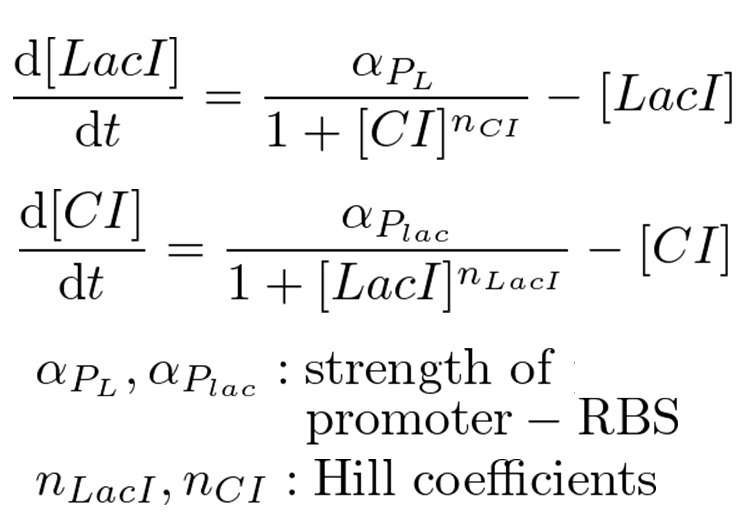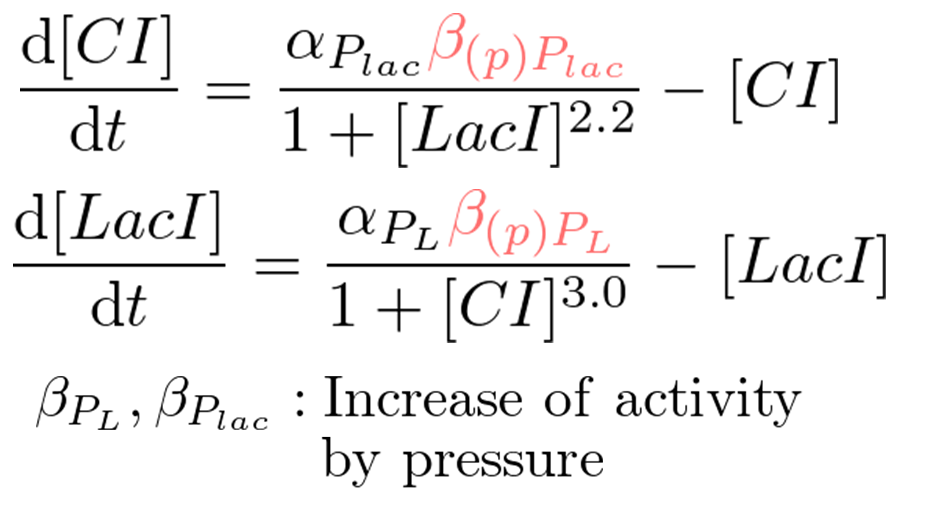Team:Tokyo Tech
From 2008.igem.org
(→Result ~ E. coli in the touch display ~) |
(→3 Touch display) |
||
| Line 134: | Line 134: | ||
[[image:Tech Pressure device2.jpg|right|400px|thumb|figure 3-1-b. Design of touch display]] | [[image:Tech Pressure device2.jpg|right|400px|thumb|figure 3-1-b. Design of touch display]] | ||
| + | {{clear}} | ||
===<font size=3>'''Basic touch display'''</font>=== | ===<font size=3>'''Basic touch display'''</font>=== | ||
Revision as of 23:42, 29 October 2008
| Main | Protcol | Parts Submitted to the Registry | Our Team | Acknowledgements |
|---|
Contents |
1 Our project
|
Our project is to create "Coli Touch"!!
|

|

|
What is "Coli touch"?
“Coli Touch” has a pressure sensitive display composed of an E. coli lawn. When you touch its display, touched section is colored.
Next I'll tell you about “Coli Touch” work system. Display of “Coli Touch” has many E. coli. When you touch this display, pressure input travel to E. coli in this display.And pressure applied E. coli expresses GFP.
Why pressure?
“Coli Touch” uses pressure as input. Then why we use pressure input? Past input way (small molecule, heat and light) are difficult to induce uniformly.
Pressurize can induce uniformly.
It's prospect of technological application in confirmatory experiment.
|
2 Pressure induction
Construction
|
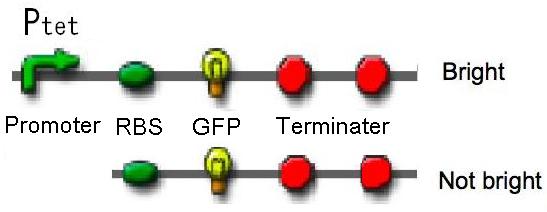
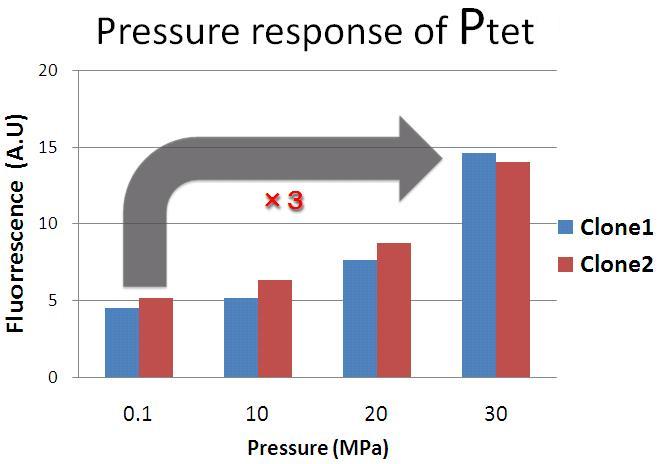
For confirming pressure-response ability of pressure-inducible promoter, we experimented under 0.1 MPa and 30 MPa pressure. We chose TetR promoter(Ptet) as pressure-inducible promoter and we constructed two plasmids - one is Ptet-GFP on pSB6, the other is promoter less-GFP on pSB6 as a negative control. |
Result ~ activity of Ptet ~
|
The result shows that Ptet activity under 30 MPa pressure is about 3 fold stronger than Ptet activity under 0.1 MPa pressure. Therefore, we confirmed that Ptet was induced under 30 MPa pressure. |
3 Touch display
Touch display (plan)
Basic touch display
|
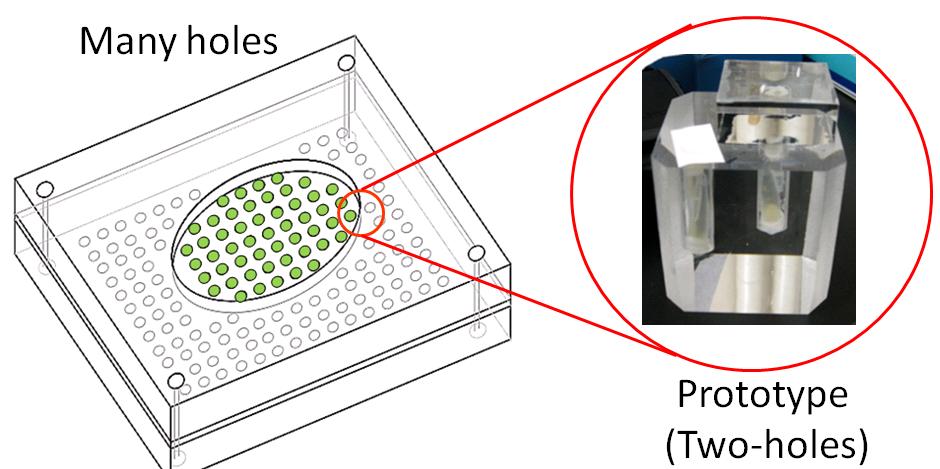
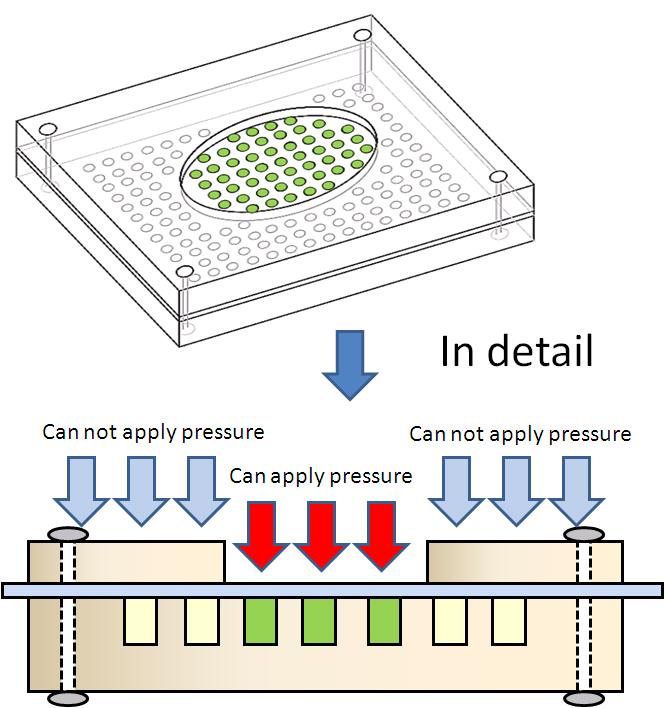
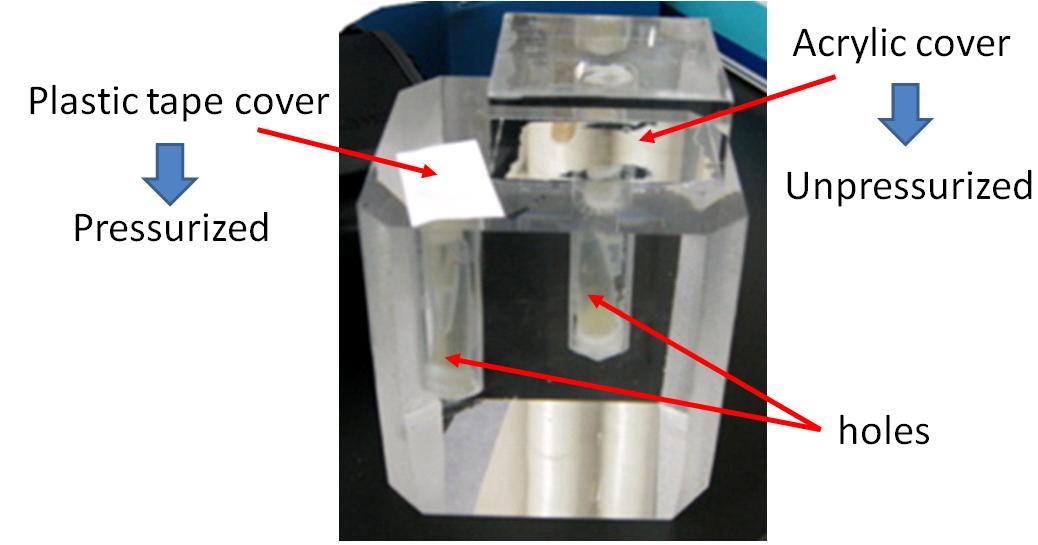
We created a basic touch display made of acrylic glasses. This touch display has two kinds of holes(show figure 3-2). Each hole contains culture medium and E. coli is cultivated in those holes. One hole (A) can be pressurized, because the hole is covered with only a plastic tape, water pressure conducts into the hole. The other (B) is not pressurized, because the hole is covered with a block made of acrylic glass, water pressure doesn’t conduct into the hole. |
How to apply pressure to “touch display”
|
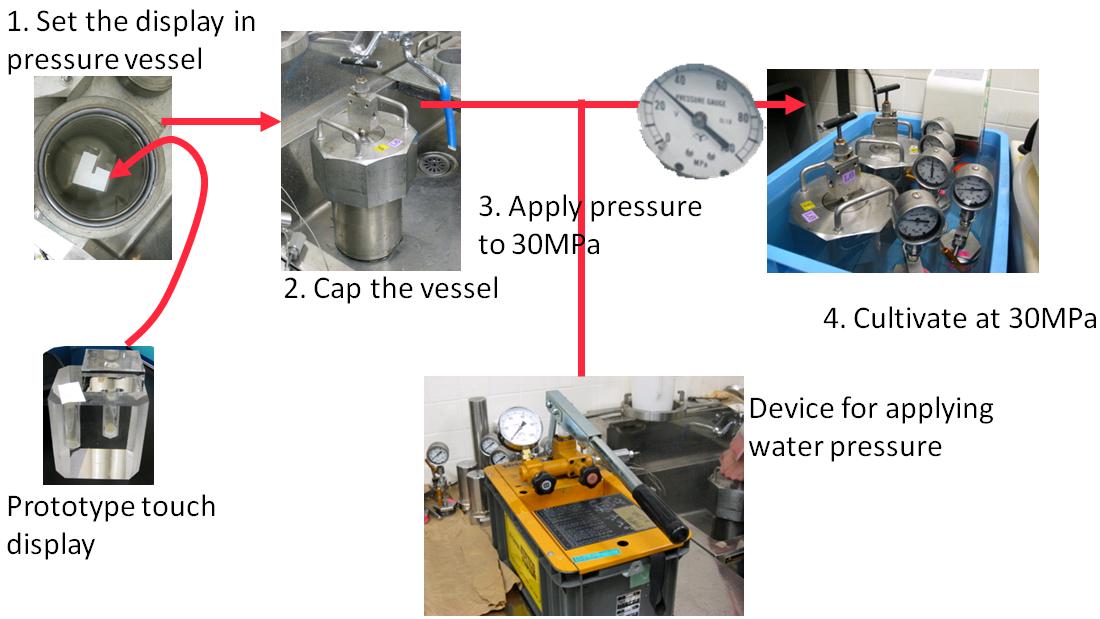
Dilute culture medium by 1% by adding fresh medium and suitable antibiotic (ampicillin; 50㎍/ml). Next, pour this culture medium into display's holes with oxygen-saturated Fluorinert (25% volume of medium). Put the display into pressure vessel filled with water (1). Next cap the vessel (2). And then apply pressure to the vessel by pressure device (4). Finally, start incubation at 37 degrees for 16h (5). |
Result ~ E. coli in the touch display ~
|
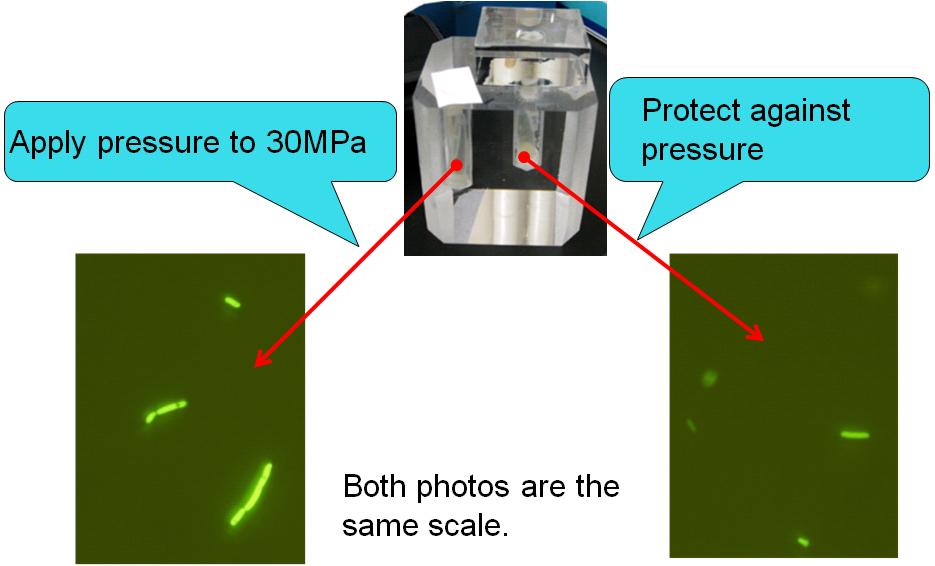
After pressurized the display, we observed the E. coli in the touch display by a fluorescence microscope. |
|
The touch display successfully regulated GFP expression in E. coli ! |
4 Low pressure-inducible promoter
Methods
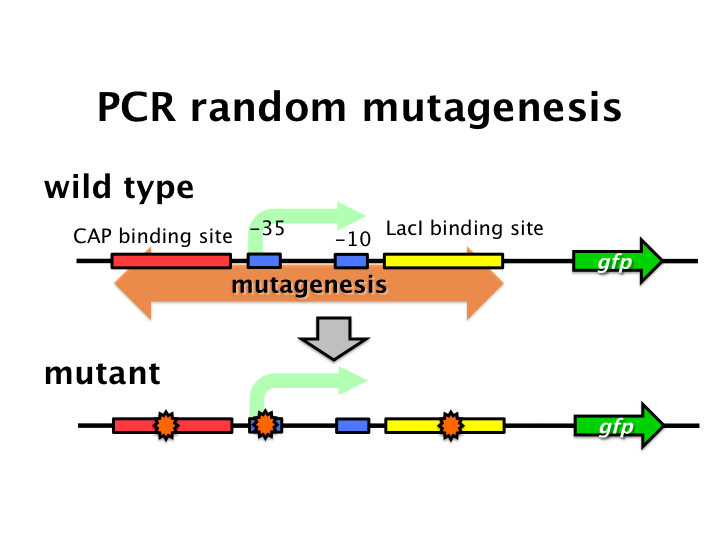
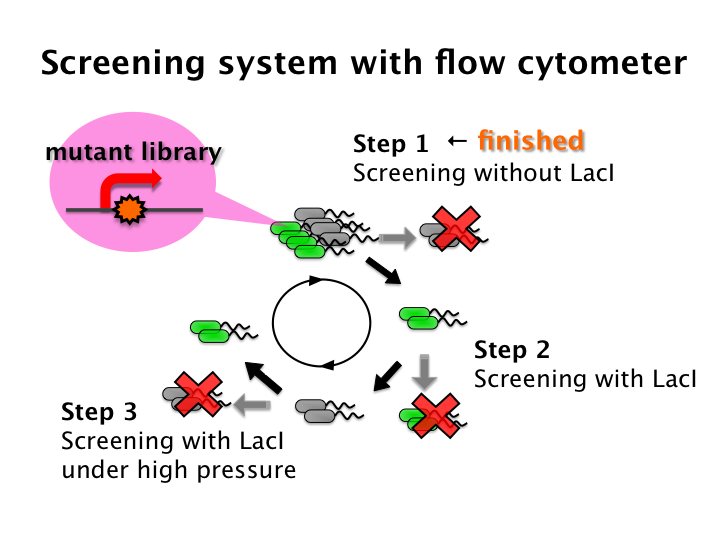
We tried to develop low pressure inducible promoter by PCR random mutagenesis to lac promoter. And we screened an E. coli library for promoters that are induced under low pressure with flow cytometry. This system is based on the ability to separate bacteria with a flow cytometer in response to expression, or lack of expression, of a fluorescent marker.
- Step 1 - Fluorescent bacteria without repressor protein were collected by a flow cytometer. This sorted pool contains bacteria bearing both constitutive and low pressure-inducible promoter.
- Step 2 - Constitutive promoter are removed with repressor protein and sorting all non-fluorescent bacteria.
- Step 3 - A final passage through under pressure with repressor protein and sorting for fluorescent bacteria removes false negatives and enriches for bacteria bearing promoter that are low pressure-inducible.
Results - Sequence and Characterization -
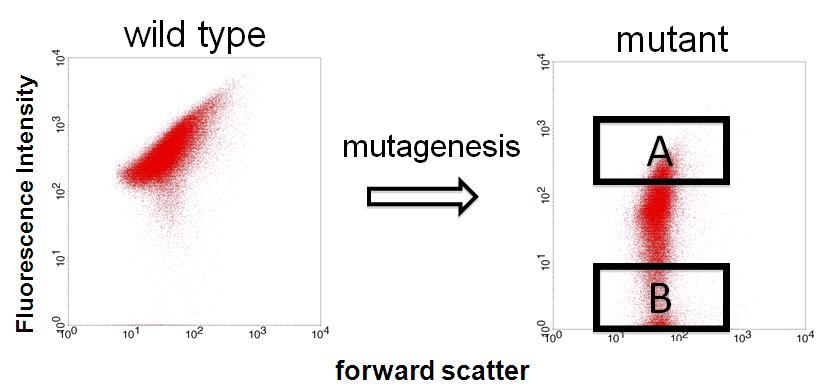
We finished step 1. Fluorescent and non-fluorescent bacteria were sorted and we characterized their promoter.
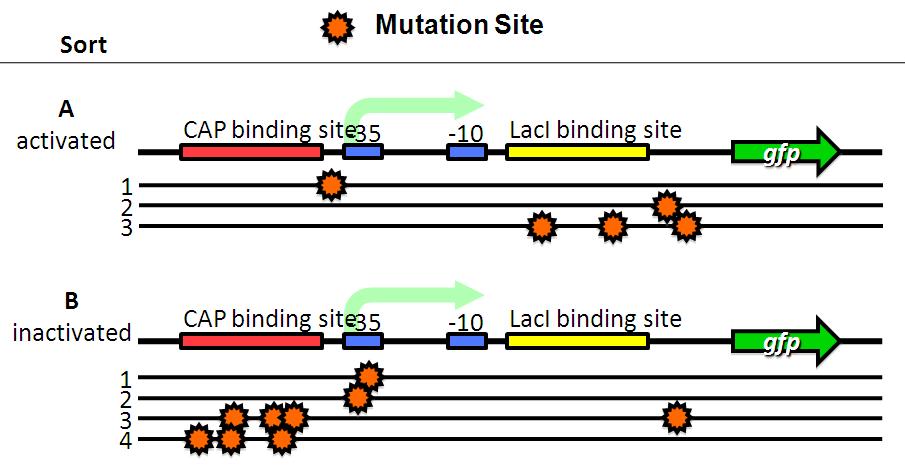
We sorted fluorescent (A) and non-fluorescent bacteria (B) with a flow cytometer. Then, we analyze these base sequences.
- A have mutations in LacI binding site or non-functional DNA.
- B have mutations in CAP binding site, -35 or non-functional DNA.
Therefore, fluorescent bacteria have no mutation in CAP binding site, -35 or -10.
Conclusion
We have successfully demonstrated that it is possible to collect objective promoter by PCR random mutagenesis and screening with a flow cytometry. So we are confident that we can screen low pressure-inducible lac promoter mutant with this methods.
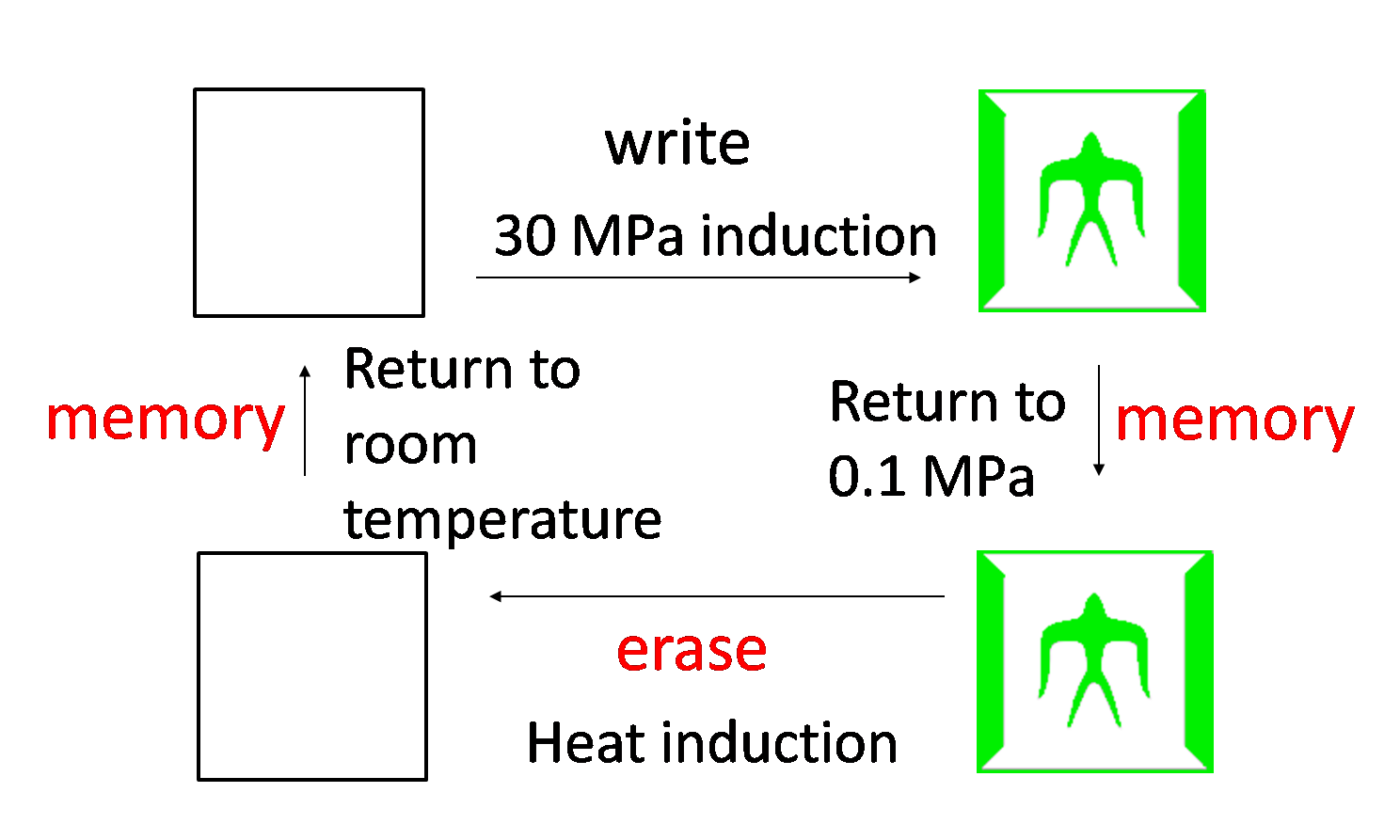
5 Write/Erase cycle
While we can implement write-function, we want to implement additionally erase-function and memory-function. Erase-function enables us to erase the painted picture, and memory-function enables us to keep the picture when we stop induction. We call these functions "Write/Erase cycle". In order to implement Write/Erase cycle, we tried to construct genetic toggle switch.
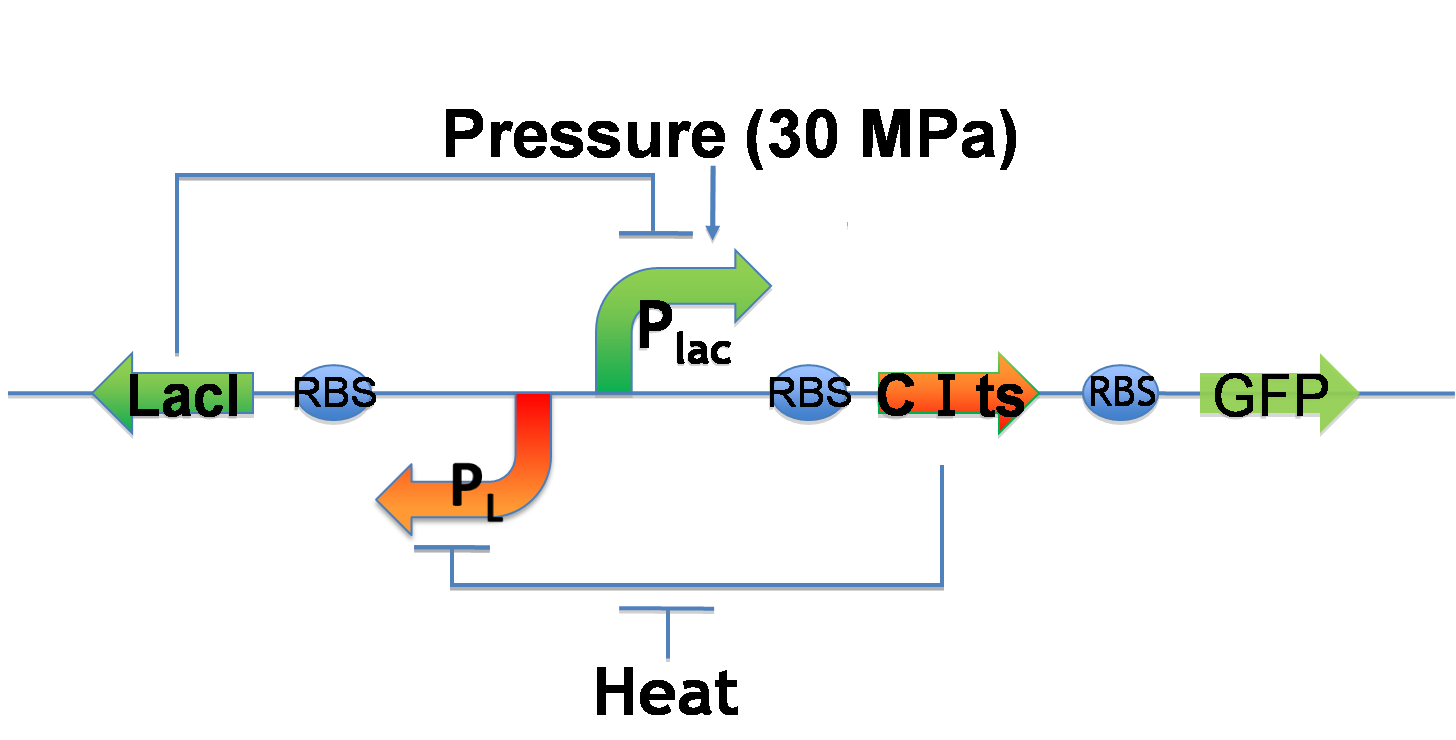
Genetic toggle switch to implement Write/Erase cycle
- Write-function
- 30 MPa pressure activates Plac.
- Plac expresses CI and GFP.
- CI represses PL and causes low LacI expression.
- Low LacI expression increases Plac activity. ⇒ Bright!!
- Erase-function
- The heat activates PL.
- PL expresses LacI.
- LacI represses Plac.
- Therefore, GFP expression decreases.
Mathematical model
Why did we use mathematical model?
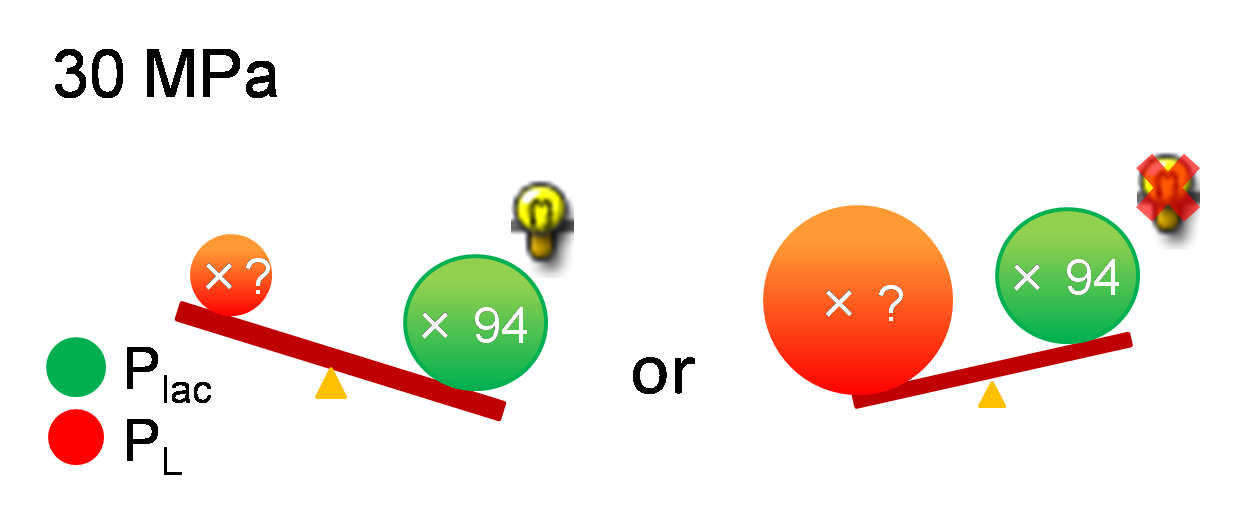
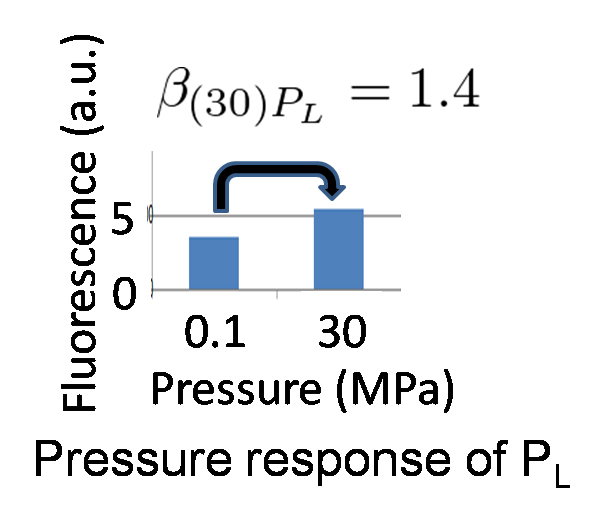
As mentioned above, it is known that Plac is activated 94.0-fold under 30 MPa while we don't know the increase of PL strength under 30 MPa. If PL is activated too much, Plac activity may be weaker than PL activity and we can't implement write-function. So, how much is the range of the increase of PL activity under 30 MPa so as to become advantageous to Plac? To know this range, we need to use mathematical model.
Classical toggle switch model
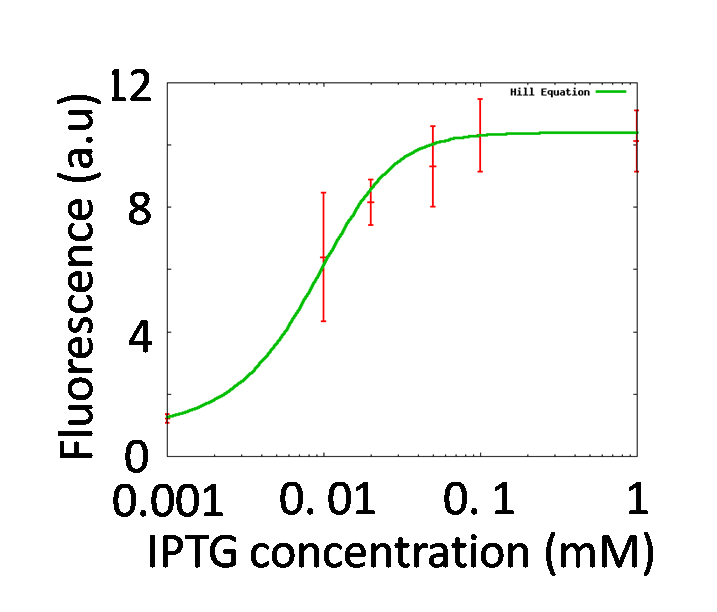
Our mathematical model under atmospheric pressure is equal to a classical toggle switch model shown in figure 5-4, where nCI is the cooperativity of repression of the lambda promoter nLacI is the cooperativity of repression of the lac promoter αPL is the effective rate of synthesis of LacI and αPlac is the effective rate of synthesis of CI. nCI and nLacI are called "Hill coefficient". αPL and αPlac depend on strength of promoter-RBS, and are adjustable. strength of promoter-RBS which are adjustable. We need to identify value of nCI and nLacI respectively. But we fortunately know nCI = 3.0 (T. Tian et al., 2006). So, we measured fluorescence intensity various IPTG concentration to identify nLacI.
Identification of nLacI
By testing how LacI represses the lac promoter, Hill coefficient of lac promoter should be decided. In order to adjust effective concentration of LacI, IPTG was added.
GFP fluorescence intensity was enhanced in an IPTG-dose dependent manner. It indicates that the LacI repression was getting weaker by adding IPTG. We formulated Hill function fitting the data shown in figure 5-5, and the characteristics of the lac promoter expressed in Hill function was determined. Finally, we obtained nLacI = 2.2.
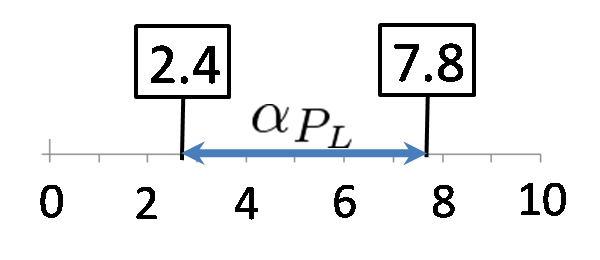
Conditions for bistability
We simulated the range of αPL in which a toggle switch model is bistability. Here, we set αPlac = 3.0. The result is below.
Pressure model
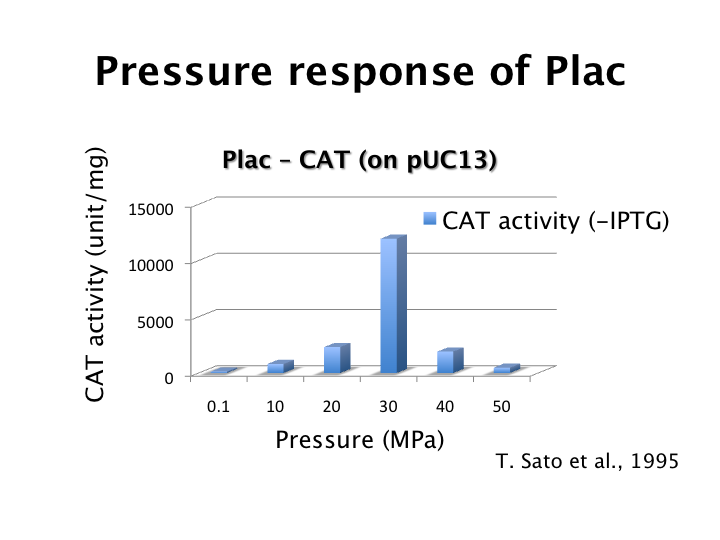
We proposed pressure model which has additional parameters to the classic toggle switch model. These parameters are the increase of activity by pressure (β(p)Plac or β(p)PL). Under atmospheric pressure (0.1 MPa), β(0.1)Plac = 1.0 and β(0.1)PL = 1.0. On the other hand, under 30 MPa, β(30)Plac = 94 and β(30)PL is not known.
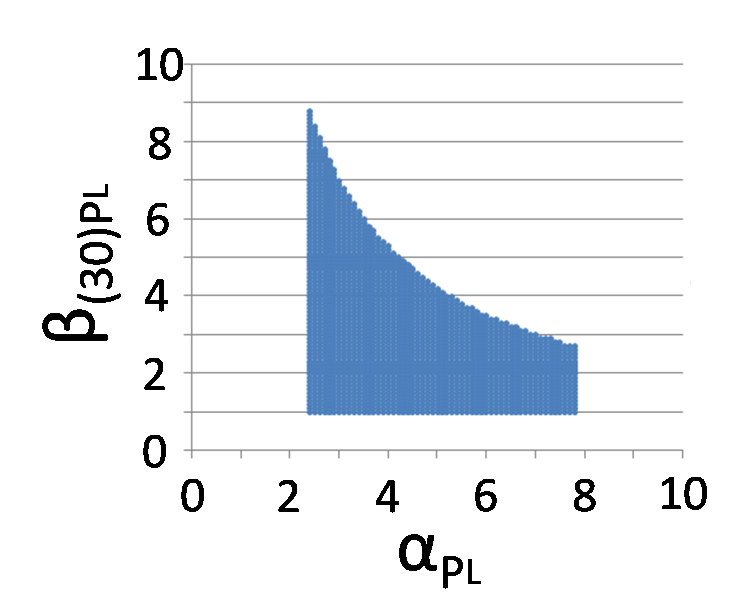
In order to implement write-function, we need the transit of the system from bistability to monostable (Plac is stronger than PL) by 30 MPa pressure. Therefore, we calculated the range of β(30)PL which satisfies the above condition.
The feasibility of implementation of Write/Erase cycle
According to the result of simulation, we found that we can implement Write/Erase function even if PL is activated 7-fold when αPL = 3.0.
We identified β(30)PL = 1.4 by our experiment under 30MPa pressure (figure 5-6). Therefore, we can implement Write/Erase cycle if we choose an appropriate PL - RBS strength.
 "
"