Team:University of Lethbridge/Notebook/GeneralLabOctober
From 2008.igem.org
Back to The University of Lethbridge Main Notebook
Contents |
October 1, 2008
Roxanne
-Inactivated the Enzymes in the morning
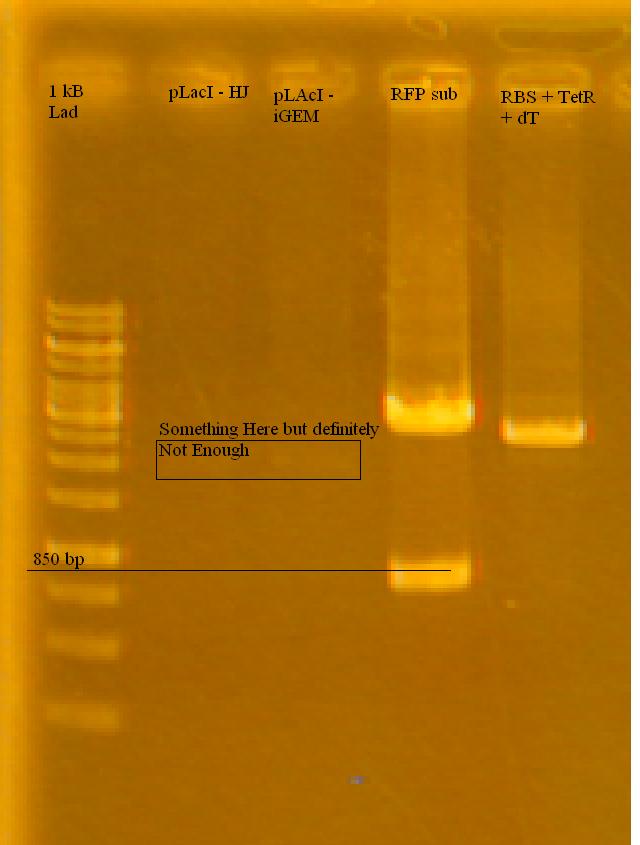
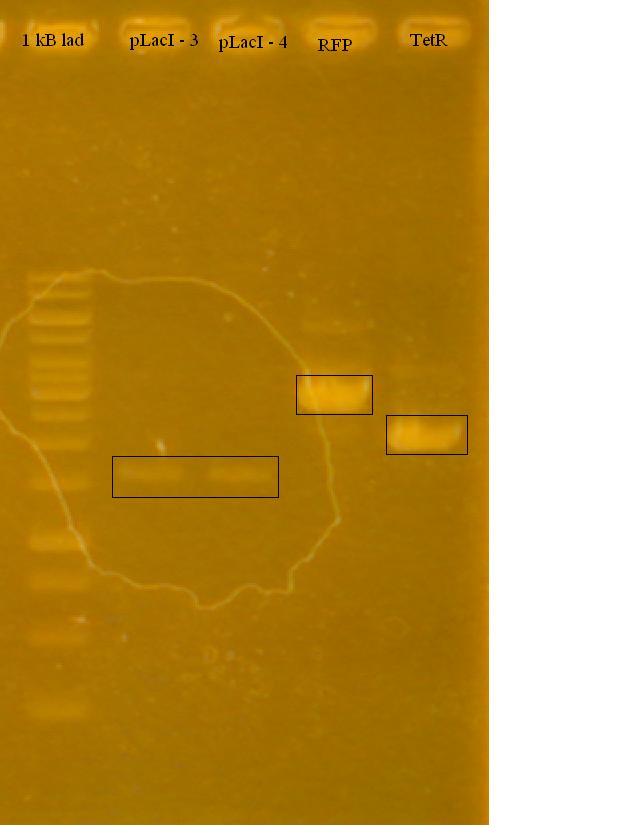
-Ran a gel of the tested pLacI, along with the previously cut RFP and TetR in the afternoon on 1% Agarose Gel in TAE.
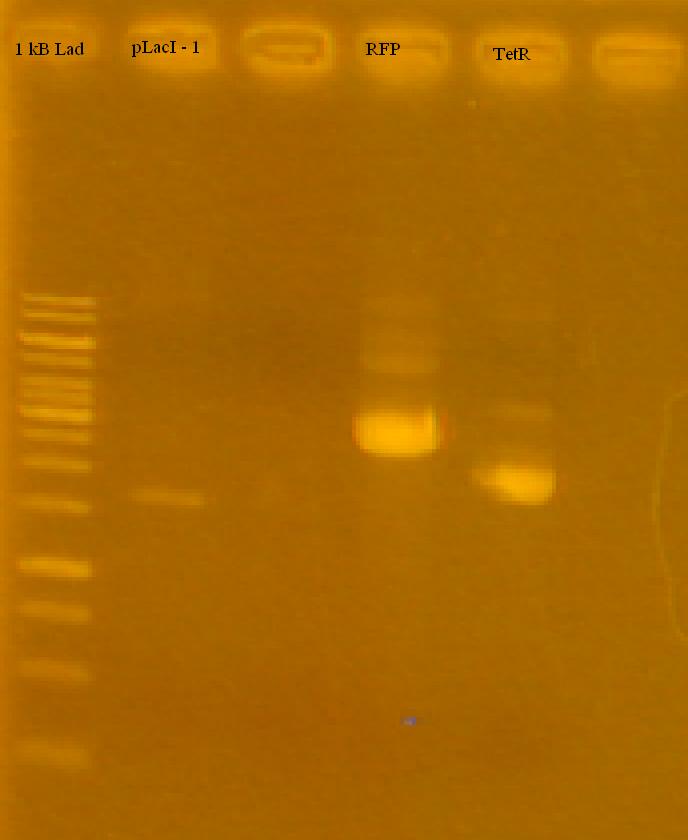
-the iGEM pLacI had a faint band, RFP looks good (half of it cut), TetR looks like it either didn't cut at all, or only cut once. Only used 2 uL for each sample, will try running with more.
October 4, 2008
Roxanne
-Reran the pLacI tests, RFP and TetR on a 1% Agarose Gel in TAE.
-Repicked pLacI x2, RFP and TetR colonies into LB+amp media since I've been having trouble with ligations. Brent suggested using lots of DNA in the ligation (<100 ng) to make ure that the ligation does in fact work this time.
October 5, 2008
Roxanne
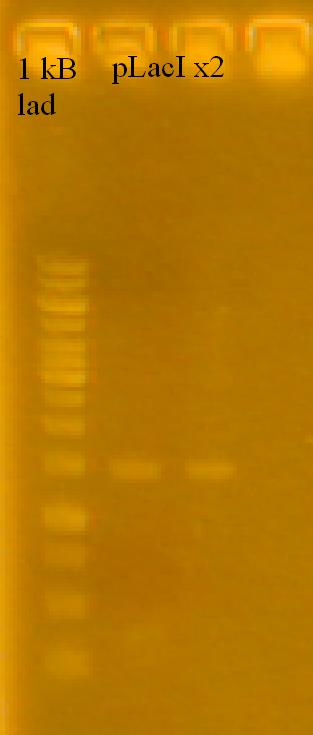
-plasmid prepped the pLacI x2, RFP and TetR subcultures. Lost one of the pLacI cultures (cells wouldn't lyse). Had some left over pLacI - 2 culture, attempted to plasmid prep those cells.
-Ran a gel of the plasmid preps.
-Restriction Digest the pLacI - 1, RFP and TetR plasmids obtained today, as well as recutting the TetR plasmid from last time, since it appears as though it didn't cut at all, or only cut once. I will be using the iGEM enzymes. Left to cut overnight at 37.0C
-picked some pLacI colonies from a different plate. Maybe I'll have better luck there. Incubate in LB+amp media at 37.0C for 15 hours.
Ocotber 6, 2008
Roxanne
-Running a gel of the parts which were Restriction Digested yesterday.
-Plasmid Prepping the new pLacI subcultures which were incubated overnight.
-Run a gel of the plasmid prep.
-Yay!!!! The Enzymes Work!!!
Christa, Munima
Made 68 LB + Amp (100 ug/mL) plates and stored them in the iGEM 4 C fridge.
Made 72 5 mL culture tubes of LB liquid media. Stored in the iGEM glass cabinet.
October 10, 2008
Munima
Objective: Isolate more plasmids for continued work on the reporter system.
Plasmid prepped 3 mL of culture for various plasmids for the reporter system: pLacI, TetR sub, pLacI-3, RFP sub, and GFP sub. They are labelled with their name and today's date in 1.5 mL microfuge tubes (50 uL of elution solution). They are stored in the "iGEM Plasmids" box in the -20 C freezer.
October 11, 2008
Roxanne
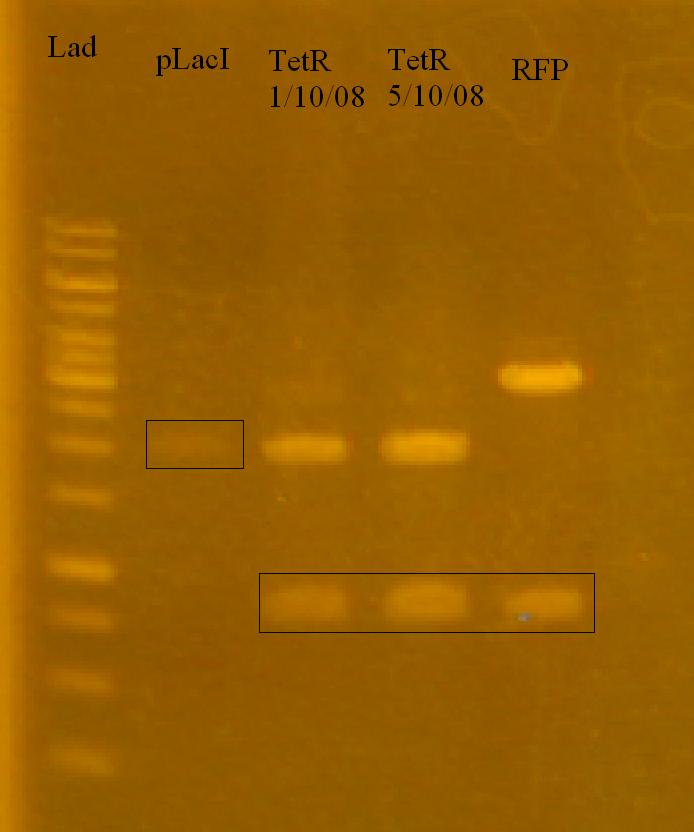
-Ran a gel of the reporter system plasmids that Munima isolated yesterday to determine to outcome.
Roxanne, Munima
Objective: Set up a Restriction Digest of the Reporter System Parts and xylE (for metabolic pathway). pLacI is cut with SpeI and PstI, RFP sub and TetR sub are cut with XbaI and PstI.
For xylE, TetTsub and RFP sub:
-4 uL of 10x Tango Buffer -2 uL of XbaI -2 uL of PstI -2 uL ddH2O -30 uL DNA
For pLacI and pLacI-3:
-4 uL of 10x Tango Buffer -2 uL of XbaI -2 uL of SpeI -2 uL ddH2O -30 uL DNA
Left in water 37 C water bath overnight.
October 12, 2008
Roxanne
-Ran a gel of the restriction digested reporter system parts on 1% Agarose in TAE at 100V for 25 minutes.
October 13, 2008
Munima, Roxanne
Objective: Another restriction digest of reporter system parts, riboswitch and CheZ with XbaI and SpeI.
For (DH5a) pzero+CheZ (Spet 30/08), RS3 (Oct. 1/08), and RS6 (Oct. 1/08)[30 uL reaction]:
-3 uL of 10x Tango Buffer -1 uL XbaI -1 uL SpeI -10 uL ddH2O -15 uL DNA
For GFP sub1 [50 uL reaction]:
-5 uL of 10x Tango Buffer -2 uL XbaI -2 uL SpeI -11 uL ddH2O -30 uL DNA
Left in 37 C water bath overnight.
October 24, 2008
Munima, Christa
Transformed
- BL21 + pUC19 - RP1616 + pTopp - GFP complete + RP1616 - DH5a + pUC19
October 25, 2008
Christa & Munima
Gel extracted pSB1A2 (Xba, Pst) with MiniElute Gel extraction kit. Ran gel after extraction for quantification- 1 uL DNA loaded.
 "
"