Team:University of Ottawa/Project
From 2008.igem.org
Contents |
Overall project
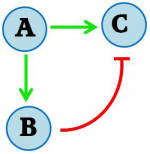
PULSATE GENE EXPRESSION: Gene expression in cells is a dynamic process in which transient responses are often observed. This can be conceptualized as a “pulse” of protein concentration. Achieving such a pulse involves a complex network of activities, which makes it difficult to elucidate the mechanisms in naturally occurring systems. Nevertheless, simple synthetic networks can be created to model this behaviour. We are working on a feed-forward regulatory motif to generate a pulsate expression of a protein of interest, in the model organism Saccharomyces cerevisiae. The general feed-forward mechanism is illustrated in Figure 1A. Upon stimulation (A), a combinatorial promoter is turned on, resulting in expression of the protein of interest (C). The stimulation also turns on another promoter encoding a repressor protein (B), which subsequently acts on the first combinatorial promoter to turn off expression of the protein of interest. Thus, a pulse in protein of interest concentration is achieved.
CELL-TO-CELL COMMUNICATION:
An important aspect of naturally occurring biological systems is the cell-to-cell communication that triggers the biomolecular responses in multicellular settings. Our pulse generator system uses signalling elements from Arabidopsis thaliana that have been implemented in yeast [Ref: Chen & Weiss, 2005], to induce the feed-forward network. One population of cells send the signal molecule cytokinin isopentenyladenine (IP), while another population receives the signal via the cytokinin receptor AtCRE1. In combination, the engineered cells receive the IP signal, which induces the pulsate expression of the protein of interest.
OSCILLATORY DYNAMICS:
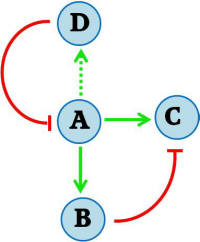
After the pulsate protein expression event, the final state of the system consists of a repressed protein of interest. It is desirable to regenerate the system so that it is sensitive to the provided stimulus again – i.e., so that the protein of interest is not repressed. This can be done by destruction of the original inducing signal molecule. Cytokinin dehydrogenase is an enzyme from A. thaliana that degrades the IP signal molecule, and has also been implemented in S. cerevisiae [Ref: Frebortova et al., 2007]. This enzyme is added to the feed-forward network in the manner illustrated in Figure 2B. Cytokinin dehydrogenase (D) is turned on slowly, and eventually inhibits the stimulus signal (A). With the signal degraded, the repressor protein (B) is no longer produced. With normal protein, any repressor protein present in the cell will inevitably be degraded. Finally, with neither the signal (A) nor the repressor (B) present, the system returns to its initial state.The two populations of sender and receiver cells can be grown together. The inducing IP signal is continually supplied to the receiver cells, which would in turn go through cycles of IP stimulation and degradation. Thus oscillatory dynamics of pulsate protein expression can be accomplished.
APPLICATIONS:
A pulse-based protein expression system implemented in the eukaryotic Saccharomyces cerevisiae has potential applicability in large-scale protein production. This method tackles the problems that have surfaced in the use of genetically modified organisms for protein production in industrial, pharmaceutical, and research settings.
Typically engineered microorganisms have been grown in bioreactors, followed by extraction and purification of the protein. However, when the protein of interest is excessively large or toxic to the cell, synthesis by the organism is difficult. Often, a small subpopulation of cells emerges, in which the protein of interest is not produced. This subpopulation soon dominates due to positive selective pressure, and the use of the bioreactor becomes less effective with time, requiring a “restart”. With our pulse generator protein expression system, the proteins are only produced in short pulses, reducing the burden on the cells, and preventing toxic accumulation. These engineered cells can thus be resilient in a bioreactor setting, making them ideal for large-scale protein production applications.
 "
"