Team:University of Washington/Project
From 2008.igem.org
(→Yeast Shuttle Vector) |
|||
| Line 25: | Line 25: | ||
| - | + | [1] Sprague, George F., and Jack A. Heinemann. “Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast.” Nature. Institute of Molecular Biology. University of Oregon. 340. 20 Jul 1989. | |
| - | + | [2] Cashmore, Annette M., Steven Bates, and Brian M. Wilkins. “IncP Plasmids Are Unusually Effective in Mediating Conjugation of Escherichia coli and Saccharomyces cerevisiae: Involvement of the Tra2 Mating System.” Journal of Bacteriology. Dec 1998. pp 6538-6543. | |
Revision as of 18:30, 30 July 2008
Contents |
Trans-Kingdom Genetic Transfer
Bacteria exhibit a means of transferring genetic material separate from traditional generational inheritance. This phenomenon of horizontal gene transfer via bacterial machinery—termed conjugation—contrasts in many aspects with traditional heritable genetics. Specifically, the mechanism of bacterial conjugation does not rely on the transfer of genetic information from parent to progeny. Instead, a unique type of plasmid—called a mobile plasmid—induces the replication and transmission of itself and other plasmids between bacteria via direct cell contact. The genes on the mobile plasmid encode for a suite of conjugative bacterial proteins, including a sex pilus nanotube that connects the bacteria and accommodates the transmission of DNA. In this manner, bacteria can accept and express genes separate from their chromosomal DNA. While this mode of genetic transfer is fascinating on many levels, including its possible role in evolution and phylogenic discrepancies, it has the potential to be utilized as an invaluable mechanism for genetic alteration.
It has been demonstrated that bacteria can transfer genetic material across kingdoms to the eukaryote yeast, an organism incredibly evolutionarily distant from any bacterial species [1]. Unlike sexual reproduction, which requires great genetic similarity between mating partners, it would appear that conjugation provides a means for transferring genetic material across vast evolutionary expanses. Our goal is to engineer E. coli to perform trans-kingdom conjugation under the presence and absence of specific environmental signals. However, in order to prevent the genetic circuitry from being “evolved out,” the yeast plasmid will confer some adaptive function to the yeast. Eventually, the yeast will be engineered to produce some beneficial product for E. coli, and the two species will live symbiotically, exchanging DNA and cellular products necessary for survival. In this way, there will be a mutualistic interaction between the yeast and E. coli, and it is hoped that the functional integrity of the genetic circuitry will remain intact.
The modular design for trans-kingdom genetic transfer could be adapted for use with various organisms under different circumstances. However, our particular design is intended for a scenario where E. coli and yeast are in close proximity, the yeast cannot naturally digest lactose, lactose is the primary food source, and the E. coli possess a transferrable plasmid containing genes for lactose digestion. Because expending resources on lactose machinery in the presence of the more readily usable glucose would be disadvantageous, in the presence of lactose, and the absence of glucose, E. coli will produce the LuxR protein. The yeast will be engineered to produce the quorum sensing molecule, AHL. When the AHL diffuses to the LuxR proteins contained in E. coli, the production of conjugation machinery will be activated, and genetic material will be transferred to yeast. Pragmatically, this schema for trans-kingdom genetic transfer could be used to modify and engineer organisms which are normally difficult to access or operate on, and also as a means of delivering functional DNA for gene therapy. Additionally, this design may serve as an entry point for more complex, multi-organism systems.
Bacterial Conjugation
The bacteria will then build the conjugation machinery, in response to the AHL produced from the yeast. This is being worked on in two separate ways on two different genes.
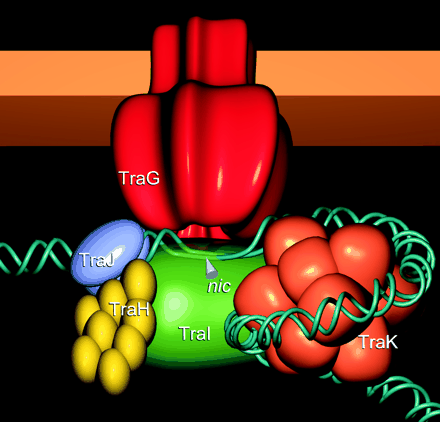
Two major genes in regulating IncP conjugation are KorA, a global regulation located on the RP4 plasmid, and TrbA, a gene that produces a transcriptional factor that controls the transcription of the TrbB operon, which contains the essential genes for building the conjugation pili and other parts of the conjugation tube.
Illustration of Tra1 Conjugation Machinery
Schröder, G., S. Krause, E. L. Zechner, B. Traxler, H. J. Yeo, R. Lurz, G. Waksman, and E. Lanka. 2002. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J. Bacteriol. 184:2767-2779. [1].
Yeast Shuttle Vector
One of the sub-projects involved in our controlled trans-kingdom conjugation project is the construction of a yeast shuttle vector. This plasmid will be stored and maintained in E. coli, and transferred to yeast via conjugation. Susbsequently, the plasmid will also be stored and maintained in yeast cells. Therefore, this yeast shuttle plasmid will have a bacterial origin of replication, a stable yeast origin of replication, a conjugative origin of transfer for RP4 mobilization, two genes which confer ampicilin and tetracycline resistance, a gene which imparts the ability to synthesize leucine, and finally the lac operon, which bestows the ability to digest lactose. A preliminary version of this plasmid, termed pAC88, has been created during prior research [2]. This pAC88 plasmid is a modified version of standard yeast plasmid YEp13; the origin of transfer site, OriT, from mobile plasmid RP4 was inserted into YEp13.
Functional pAC88 plasmids have been obtained from researchers at Leicester, which is a standard yeast cloning vector, YEp13, with an origin of transfer for RP4. The insertion of the RP4 OriT interrupts the gene for tetracycline resistance on YEp13, which is verifiable by transformed cells' sensitivity to tetracycline. In order to determine the efficacy of conjugation, S. cerevisiae was grown with E. coli in leucine-deficient media. Because pAC88 contains a gene which confers the ability to synthesize leucine, a strain of yeast which is normally auxotrophic for this amino acid was used. Successful conjugations resulted in yeast colonies that contained leucine.
There still remains the task of inserting the adaptive gene into the shuttle plasmid. Kluyveromyces lactis, a species of milk-yeast, posesses a region of chromosomal DNA that encodes for lactose digestion machinery. LAC4 and LAC12, two genes that encode for yeast galactosidase and lactose permease, respectively, are located near each other on the chromosome, and are divergently transcribed. Primers will be designed which are complementary to the transcriptional endpoints of LAC4 and LAC12, with hanging ends that correspond to the restriction enzyme HinDIII, an exploitable restriction site present in pAC88. A restriction digest on pAC88 and ligation with the LAC4-LAC12 cassette will insert the lactose digestion machinery into the yeast shuttle plasmid. By growing transformed yeast on a medium containing the molecular species X-gal, it will be determined whether the cassette has been successfully inserted if the yeast colonies appear blue.
[1] Sprague, George F., and Jack A. Heinemann. “Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast.” Nature. Institute of Molecular Biology. University of Oregon. 340. 20 Jul 1989.
[2] Cashmore, Annette M., Steven Bates, and Brian M. Wilkins. “IncP Plasmids Are Unusually Effective in Mediating Conjugation of Escherichia coli and Saccharomyces cerevisiae: Involvement of the Tra2 Mating System.” Journal of Bacteriology. Dec 1998. pp 6538-6543.
UW Lab Links
Protocols
BioBrick Parts Used
UW Glycerol Stocks
| Home | The Team | The Project | Parts Submitted to the Registry | Modeling | Notebook |
|---|
 "
"