Team:University of Lethbridge/Notebook/GeneralLabSeptember
From 2008.igem.org
Munima.alam (Talk | contribs) m (link to go back to the Main notebook) |
Munima.alam (Talk | contribs) m (spacing) |
||
| (8 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
[[Team:University_of_Lethbridge/Notebook|Back to The University of Lethbridge Main Notebook]] | [[Team:University_of_Lethbridge/Notebook|Back to The University of Lethbridge Main Notebook]] | ||
| + | |||
| + | ===August 31, 2008=== | ||
| + | ====Nathan Puhl, Roxanne==== | ||
| + | -Transformed DH5a cells with the biobrick part R0011 (pLacI) obtained from the 2007 iGEM parts. Plated on LB + amp agar plates. | ||
| + | |||
| + | |||
| + | ===September 1, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -Picked 3 representative colonies and incubated them in LB+amp media overnight at 37.0C. | ||
| + | |||
| + | |||
| + | ===September 2, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -plasmid prepped the pLacI cells which had been incubated the night before. Ran plasmids on a 1% Agarose Gel at 100V for 27 minutes | ||
| + | |||
| + | ====Nathan Puhl==== | ||
| + | -Visualized the Gel and Glycerol Stocked the Cells. | ||
| + | |||
| + | |||
| + | ===September 3, 2008=== | ||
| + | ====Roxanne, Nathan Puhl==== | ||
| + | -Performed a Restriction Digest of I13504 (RBS+GFP+dT) and R0011 (pLacI recombinant) | ||
| + | |||
| + | -10 uL template DNA | ||
| + | -0.5 uL Restriction Enzyme #1 | ||
| + | -0.5 uL Restriction Enzyme #2 | ||
| + | -2 uL NEB 2 | ||
| + | -7 uL ddH2O | ||
| + | _________ | ||
| + | 20 uL Reaction overnight at 37.0C | ||
| + | |||
| + | ===Mid-September, 2008=== | ||
| + | ====Roxanne, Nathan Puhl==== | ||
| + | -Have been working on Cloning the Reporter System. Transformation worked, however, the lack of Red colonies on the RFP plates and sub-culture have led us to believe that something didn't work somewhere along the way. | ||
| + | |||
| + | |||
| + | ===September 13, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -Streaked Plates of RFP sub, TetR sub and pLacI | ||
| + | |||
| + | |||
| + | ===September 14, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -Picked colonies from the RFP sub, TetR sub and pLacI plates. Incubating overnight at 37.0C in LB media + amp. | ||
| + | |||
| + | |||
| + | ===September 17, 2008=== | ||
| + | ====Nathan Puhl==== | ||
| + | -Plasmid prepped the RFP sub, TetR sub and pLacI cultures. | ||
| + | |||
| + | ====Nathan Puhl, Roxanne==== | ||
| + | -Restriction digest of the RFP sub and TetR sub with XbaI and PstI | ||
| + | -Restriction Digest of pLacI with SpeI and PstI. | ||
| + | |||
| + | |||
| + | ===September 18, 2008=== | ||
| + | ====Nathan Puhl==== | ||
| + | -Ran a 1% agarose gel of the digestion products. RFP and TetR look good, however, pLacI is running high. | ||
| + | |||
| + | |||
| + | ===September 19, 2008=== | ||
| + | ====Nathan Puhl, Roxanne==== | ||
| + | -repicked colonies from pLacI plates for subculture in LB media. | ||
| + | |||
| + | |||
| + | ===September 20, 2008=== | ||
| + | ====Nathan Puhl, Roxanne==== | ||
| + | -plasmid prepped the pLacI culture. | ||
| + | -Ran plasmids on a 1% agarose gel. | ||
| + | -Oops, sub-culture it again. | ||
| + | |||
| + | |||
| + | ===September 21, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -plasmid prepped the pLacI culture. | ||
| + | -Ran the gel of the plasmid prep products | ||
| + | |||
| + | [[Image:PLacIprep.jpg|350 px]] | ||
| + | |||
| + | |||
| + | ===September 22, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -Gel Extraction of pLacI x2, RFP sub, and TetR sub | ||
| + | |||
| + | [[Image:extraction.jpg|350 px]] | ||
| + | |||
| + | -Ran a gel of the extraction products, however, no product appears. | ||
| + | |||
| + | |||
| + | ===September 23, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -Ran the gel of the extraction products again. | ||
| + | -1 uL of DNA on a 1% agarose gel @ 100V for 25 minutes, with a 1kb ladder. | ||
| + | -The Kit ate my DNA again!! | ||
| + | -Repicked colonies from the plate and incubated in LB+amp media overnight @ 37.0C | ||
| + | |||
| + | |||
| + | ===September 24, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -Took the tubes out of the shaker and put in fridge to be plasmid prepped at a later date. | ||
| + | |||
| + | |||
| + | ===September 27, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -plasmid prepped the pLacI, RFP and TetR genes | ||
| + | -Ran the plasmids on a gel. RFP and TetR look good, pLacI is a little wonky, not comforteble with it | ||
| + | -Setup a Restriction Digest of RFP and TetR, using XbaI and PstI. | ||
| + | -Repicked te pLacI colony and incubated in LB+amp overnight @ 37.0C | ||
| + | |||
| + | |||
| + | ===September 28, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -Plasmid prepped the pLacI gene and put te plasmid in the freezer | ||
| + | -Inactivated the enzymes from the RFP and TetR double digest | ||
| + | |||
| + | |||
| + | ===September 30, 2008=== | ||
| + | ====Roxanne==== | ||
| + | -Testing the Enzymes we received from Fermentas which were sent without ice on the pLacI gene | ||
| + | -Cut Half of my plasmid prep with the iGEM SpeI and PstI, and the other half with H-J's PstI and the iGEM SpeI. | ||
Latest revision as of 02:30, 30 October 2008
Back to The University of Lethbridge Main Notebook
August 31, 2008
Nathan Puhl, Roxanne
-Transformed DH5a cells with the biobrick part R0011 (pLacI) obtained from the 2007 iGEM parts. Plated on LB + amp agar plates.
September 1, 2008
Roxanne
-Picked 3 representative colonies and incubated them in LB+amp media overnight at 37.0C.
September 2, 2008
Roxanne
-plasmid prepped the pLacI cells which had been incubated the night before. Ran plasmids on a 1% Agarose Gel at 100V for 27 minutes
Nathan Puhl
-Visualized the Gel and Glycerol Stocked the Cells.
September 3, 2008
Roxanne, Nathan Puhl
-Performed a Restriction Digest of I13504 (RBS+GFP+dT) and R0011 (pLacI recombinant)
-10 uL template DNA -0.5 uL Restriction Enzyme #1 -0.5 uL Restriction Enzyme #2 -2 uL NEB 2 -7 uL ddH2O _________ 20 uL Reaction overnight at 37.0C
Mid-September, 2008
Roxanne, Nathan Puhl
-Have been working on Cloning the Reporter System. Transformation worked, however, the lack of Red colonies on the RFP plates and sub-culture have led us to believe that something didn't work somewhere along the way.
September 13, 2008
Roxanne
-Streaked Plates of RFP sub, TetR sub and pLacI
September 14, 2008
Roxanne
-Picked colonies from the RFP sub, TetR sub and pLacI plates. Incubating overnight at 37.0C in LB media + amp.
September 17, 2008
Nathan Puhl
-Plasmid prepped the RFP sub, TetR sub and pLacI cultures.
Nathan Puhl, Roxanne
-Restriction digest of the RFP sub and TetR sub with XbaI and PstI -Restriction Digest of pLacI with SpeI and PstI.
September 18, 2008
Nathan Puhl
-Ran a 1% agarose gel of the digestion products. RFP and TetR look good, however, pLacI is running high.
September 19, 2008
Nathan Puhl, Roxanne
-repicked colonies from pLacI plates for subculture in LB media.
September 20, 2008
Nathan Puhl, Roxanne
-plasmid prepped the pLacI culture. -Ran plasmids on a 1% agarose gel. -Oops, sub-culture it again.
September 21, 2008
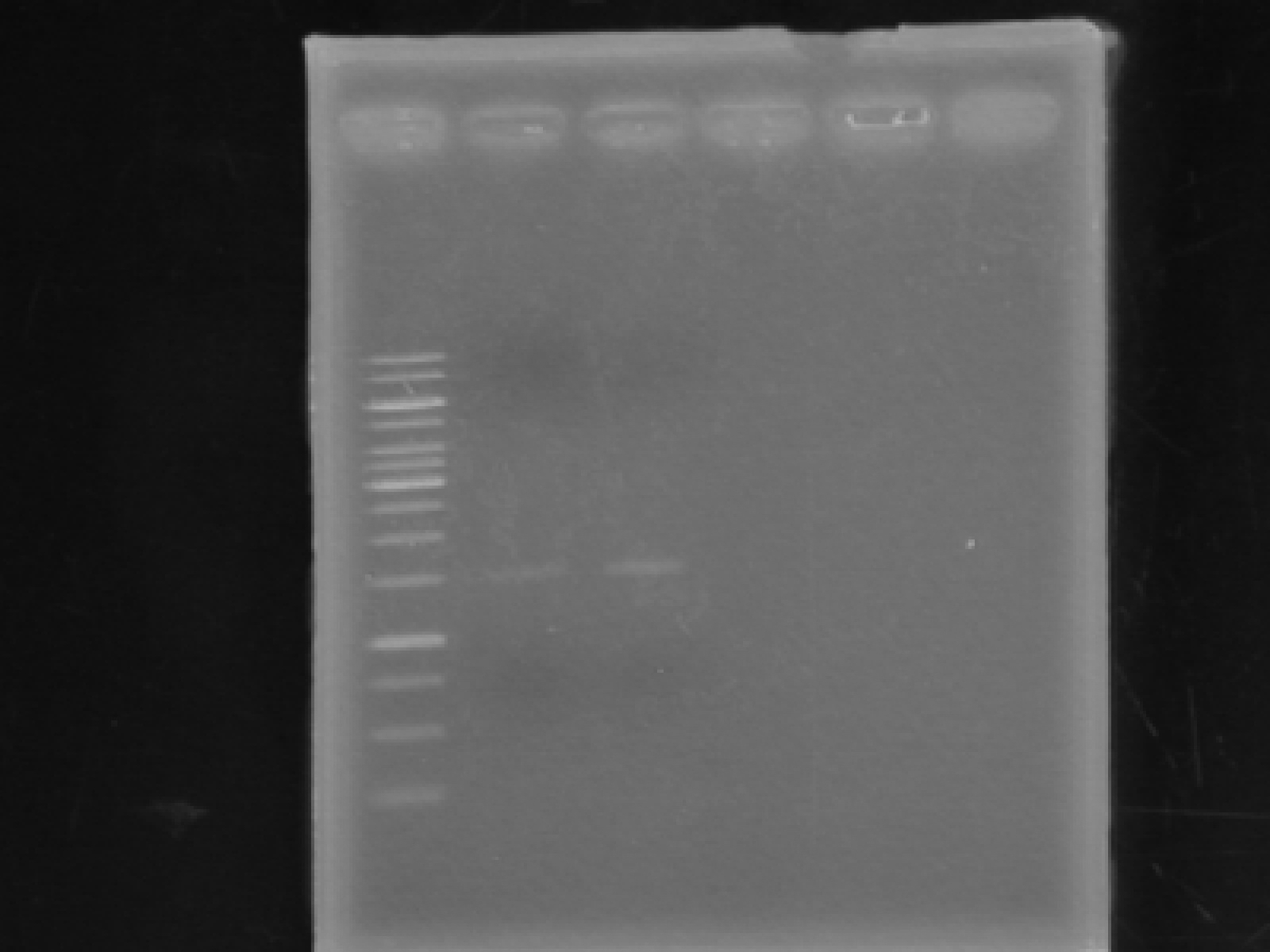
Roxanne
-plasmid prepped the pLacI culture. -Ran the gel of the plasmid prep products
September 22, 2008
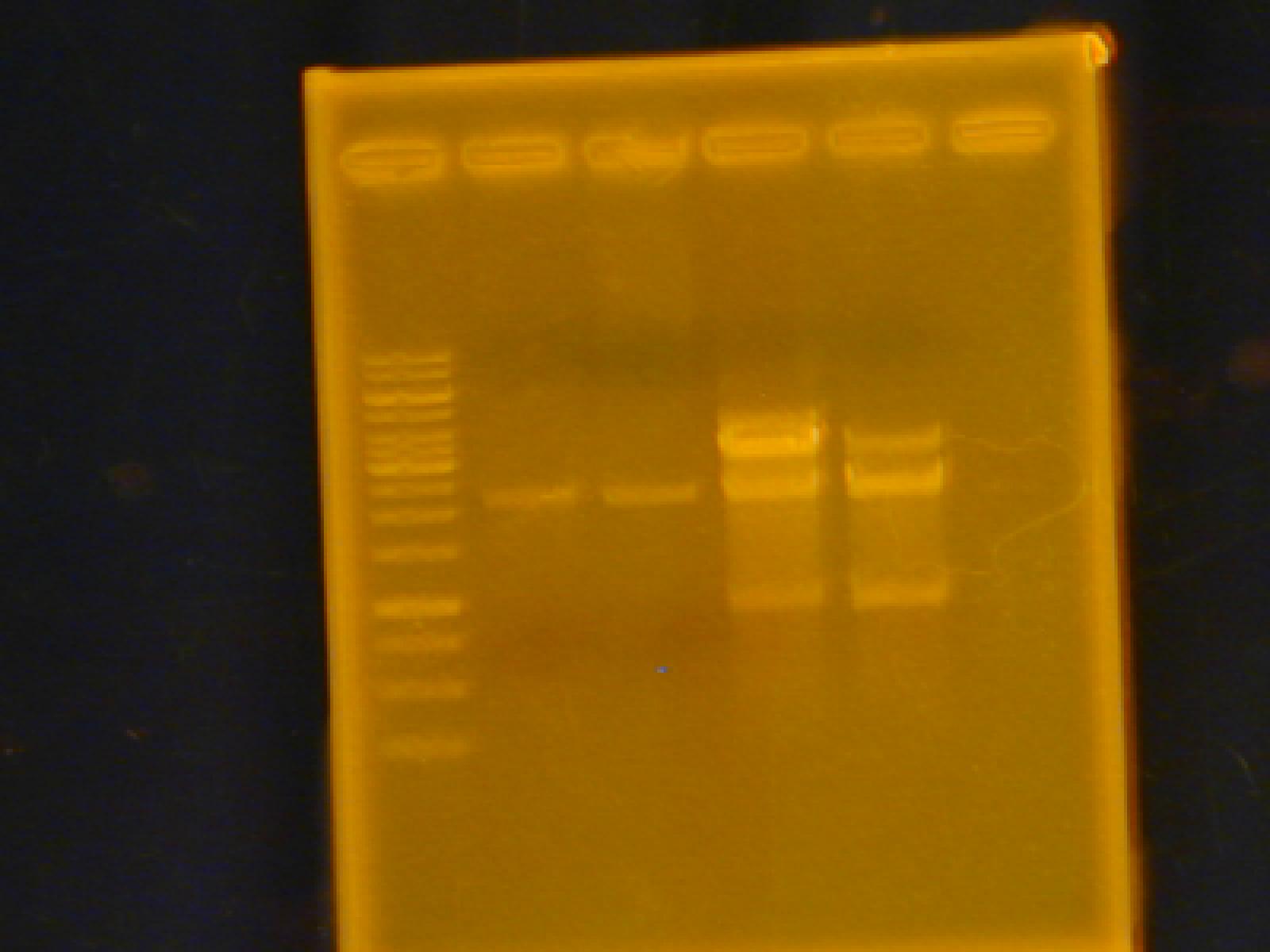
Roxanne
-Gel Extraction of pLacI x2, RFP sub, and TetR sub
-Ran a gel of the extraction products, however, no product appears.
September 23, 2008
Roxanne
-Ran the gel of the extraction products again. -1 uL of DNA on a 1% agarose gel @ 100V for 25 minutes, with a 1kb ladder. -The Kit ate my DNA again!! -Repicked colonies from the plate and incubated in LB+amp media overnight @ 37.0C
September 24, 2008
Roxanne
-Took the tubes out of the shaker and put in fridge to be plasmid prepped at a later date.
September 27, 2008
Roxanne
-plasmid prepped the pLacI, RFP and TetR genes -Ran the plasmids on a gel. RFP and TetR look good, pLacI is a little wonky, not comforteble with it -Setup a Restriction Digest of RFP and TetR, using XbaI and PstI. -Repicked te pLacI colony and incubated in LB+amp overnight @ 37.0C
September 28, 2008
Roxanne
-Plasmid prepped the pLacI gene and put te plasmid in the freezer -Inactivated the enzymes from the RFP and TetR double digest
September 30, 2008
Roxanne
-Testing the Enzymes we received from Fermentas which were sent without ice on the pLacI gene -Cut Half of my plasmid prep with the iGEM SpeI and PstI, and the other half with H-J's PstI and the iGEM SpeI.
 "
"