August
From 2008.igem.org
(Difference between revisions)
m |
|||
| (5 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
Content= | Content= | ||
<div style="font-size:18pt;"> | <div style="font-size:18pt;"> | ||
| - | <font face="Arial Rounded MT Bold" style="color:#010369"> | + | <font face="Arial Rounded MT Bold" style="color:#010369">_august</font></div> |
__NOTOC__ | __NOTOC__ | ||
<br> | <br> | ||
| Line 59: | Line 59: | ||
There again were no colonies on the plates. | There again were no colonies on the plates. | ||
| + | '''Preparing T-cells for MTT-assay''' (Normann) | ||
| + | To test the Mg2+ tolerance of the T-cells 800µl medium was given to 100µl cellsuspension and 100µl MgCl2/MgAc Solution with different concentrations.<br><br> | ||
| + | well --- Mg2+ [mM]<br> | ||
| + | 1 ------- control<br> | ||
| + | 2 ------- 12.5<br> | ||
| + | 3 ------- 8.75<br> | ||
| + | 4 ------- 6.25<br> | ||
| + | 5 ------- 5<br> | ||
| + | 6 ------- 3.75<br> | ||
| + | 7 ------- 2.5<br> | ||
| + | 8 ------- 1.25<br> | ||
<h3>Aug. 14th 2008</h3> | <h3>Aug. 14th 2008</h3> | ||
| Line 81: | Line 92: | ||
<h3>Aug. 15th 2008</h3> | <h3>Aug. 15th 2008</h3> | ||
<br> | <br> | ||
| - | There were colonies on the plates from both parts. | + | There were colonies on the plates from both parts.<br> |
| - | + | '''Splitting T-cells'''(Normann)<br> | |
| + | The cells (15ml) were spun down at 1200 rpm (5min) and resolved with 10ml PBS. Then they were spun down again and resolved in 15ml fresh medium. 5ml was given to a dish and 15ml additional medium was added. | ||
| + | <br> | ||
| + | '''MTT-assay''' Normann | ||
| + | <ul> | ||
| + | <li> centrifuging 1ml cellsuspension at 1200 rpm (5min)-> discarding the supernatant | ||
| + | <li> adding 200µl fresh medium and 50µl MTT | ||
| + | <li> waiting for 4h | ||
| + | <li> centrifuging again at 1200rpm (5min) -> discarding the supernantant | ||
| + | <li> resolving the pellet with 400µl DMSO and adding 50µl Soerensen | ||
| + | <li> incubation for 10 min | ||
| + | <li> measuring at 570nm (autozero with DMSO+Soerensen) | ||
| + | <br> | ||
| + | <table style="text-align: left; width: 100px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>Mg2+ [mM]</td> | ||
| + | <td>A (MgCl2)</td> | ||
| + | <td>A (MgAc)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>12,5</td> | ||
| + | <td>0,388 </td> | ||
| + | <td>0,284</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>8,75</td> | ||
| + | <td>0,488</td> | ||
| + | <td>0,23<td> | ||
| + | </tr><tr> | ||
| + | <td>6,25</td> | ||
| + | <td>0,568</td> | ||
| + | <td>0,217</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>5</td> | ||
| + | <td>0,328</td> | ||
| + | <td>0,22</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>3,75</td> | ||
| + | <td>0,365</td> | ||
| + | <td>0,311</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>2,5</td> | ||
| + | <td>0,255</td> | ||
| + | <td>0,295</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>1,25</td> | ||
| + | <td>0,207</td> | ||
| + | <td>0,241</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>control</td> | ||
| + | <td>0,449</td> | ||
| + | <td>0,395</td> | ||
| + | </tr> | ||
| + | </table> | ||
<h3>Aug. 17th 2008</h3> | <h3>Aug. 17th 2008</h3> | ||
| Line 148: | Line 218: | ||
<br> | <br> | ||
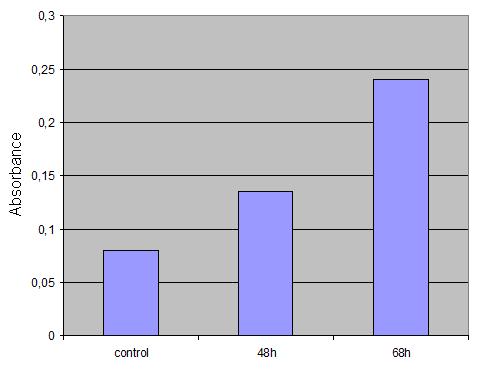
'''Harvesting cells'''<br> | '''Harvesting cells'''<br> | ||
| - | For the ONPG Test we decided to use cells after several timepoints. So I harvested cells after 48h.<br> | + | For the ONPG Test we decided to use cells after several timepoints. So I harvested cells after 48h and 68h.<br> |
| - | I washed the cells twice with PBS. Then I took 500µl PBS, lost the cells with a cell scraper and transfered it to an eppi. After centrifugation (2min 13000rpm) I replaced the PBS with 500µl 1xLysepuffer, resuspended the pellet by pipetting up and down and put it for 15 min at -80°. Then I thawed the cells, vortexed it, centrifuged again and transfered the supernatant to a fresh eppi. Then I put the supernatant at -20°.<br> | + | I washed the cells twice with PBS. Then I took 500µl PBS, lost the cells with a cell scraper and transfered it to an eppi. After centrifugation (2min 13000rpm) I replaced the PBS with 500µl 1xLysepuffer, resuspended the pellet by pipetting up and down and put it for 15 min at -80°. Then I thawed the cells, vortexed it, centrifuged again and transfered the supernatant to a fresh eppi. Then I put the supernatant at -20°.<br>Control was done with untransfected cells using the same procedure.<br> |
| + | '''Result'''<br> | ||
| + | [[Image:ONPG-Assay-Ergebnis.gif|300px]] | ||
}} | }} | ||
Latest revision as of 02:40, 30 October 2008
 "
"