Team:Hawaii/Test Competent E. Coli
From 2008.igem.org
(Difference between revisions)
(→Discussion) |
m (→Test Competent DB3.1 Cells) |
||
| (8 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | = Test Competent E. coli DH5a = | |
* Test our competent cells against Callahan competent cells | * Test our competent cells against Callahan competent cells | ||
| + | :* Competency was chemically induced by RbCl | ||
| - | + | == Methods == | |
| - | + | === Materials === | |
* Transported 50uL of competent cells from Callahan Lab on ice | * Transported 50uL of competent cells from Callahan Lab on ice | ||
* 100uL batch 1 competent cells made at OD<sub>600</sub> ~0.6 (split into two 50 uL aliquots 4-18a,4-18b) | * 100uL batch 1 competent cells made at OD<sub>600</sub> ~0.6 (split into two 50 uL aliquots 4-18a,4-18b) | ||
* 100uL batch 2 competent cells made at OD<sub>600</sub> ~0.3 (split into two 50 uL aliquots 2-17a,2-17b) | * 100uL batch 2 competent cells made at OD<sub>600</sub> ~0.3 (split into two 50 uL aliquots 2-17a,2-17b) | ||
| - | + | === Procedure === | |
# Thaw plasmid on ice | # Thaw plasmid on ice | ||
# Add 1uL plasmid pBluescript | # Add 1uL plasmid pBluescript | ||
| Line 18: | Line 19: | ||
# Plate 20 uL dilutions 1/1, 1/10, 1/100 on LB+amp to test competency | # Plate 20 uL dilutions 1/1, 1/10, 1/100 on LB+amp to test competency | ||
| - | + | == Results == | |
{| border="1" | {| border="1" | ||
|+ '''Colony Count of pBluescript Transformants''' | |+ '''Colony Count of pBluescript Transformants''' | ||
| - | ! | + | !Source DH5-α |
| - | ! | + | !Dilution |
| - | ! | + | !Colony Forming Units on Plate |
| - | ! | + | !Number of Transformed Cells per mL |
|- | |- | ||
| - | | Callahan lab|| 1:1 || Too many to count || n/a | + | | rowspan="3" align="center"|Callahan lab||align="center"| 1:1 || align="center"|Too many to count || align="center"|n/a |
|- | |- | ||
| - | | | + | | align="center"|1:10|| align="center"|Too many to count || align="center"|n/a |
|- | |- | ||
| - | | | + | | align="center"|1:100|| align="center"|201||align="center"|1,005,000 |
|- | |- | ||
| - | | 2-17 (OD600=0.3)||1:1||Too many to count||n/a | + | | rowspan="3" align="center"|2-17 (OD600=0.3)||align="center"|1:1||align="center"|Too many to count||align="center"|n/a |
|- | |- | ||
| - | | | + | | align="center"|1:10|| align="center"|334 || align="center"|167,000 |
|- | |- | ||
| - | | | + | | align="center"|1:100|| align="center"|17 <sup>[1]</sup> ||align="center"|85,000 |
|- | |- | ||
| - | | 4-18 (OD600=0.6)|| 1:1|| Too many to count||n/a | + | | rowspan="3" align="center"|4-18 (OD600=0.6)|| align="center"|1:1||align="center"| Too many to count||align="center"|n/a |
|- | |- | ||
| - | | | + | | align="center"|1:10|| align="center"|352 <sup>[2]</sup>||align="center"|176,000 |
|- | |- | ||
| - | | | + | | align="center"|1:100|| align="center"|40||align="center"|200,000 |
|- | |- | ||
| - | | 4-17 (OD600=0.6)||1:1 (negative control)||0||0 | + | | align="center"|4-17 (OD600=0.6)||rowspan="2" align="center"|1:1 (negative control)||align="center"|0||align="center"|0 |
|- | |- | ||
| - | | 2-15 (OD600=0.3)|| | + | | align="center"|2-15 (OD600=0.3)||align="center"|0||align="center"|0 |
|- | |- | ||
| - | | 4-17 (OD600=0.6)||1:1 (positive control <sup>[3]</sup>)|| Bacterial lawn||n/a | + | | align="center"|4-17 (OD600=0.6)||rowspan="2" align="center"|1:1 (positive control <sup>[3]</sup>)|| align="center"|Bacterial lawn||align="center"|n/a |
|- | |- | ||
| - | | 2-15 (OD600=0.3)|| | + | | align="center"|2-15 (OD600=0.3)||align="center"|Bacterial lawn||align="center"|n/a |
|} | |} | ||
''Notes:<br> <sup>1</sup> Fewer than 30 colonies results in a statistically inaccurate estimation of cell density. CFU should be between 30 and 300. <br><sup>2</sup> Colonies formed in one quadrant (88) were counted and multiplied by 4 to give final cell density.<br> <sup>3</sup> Positive control was plated on plain LB plates, without amp. | ''Notes:<br> <sup>1</sup> Fewer than 30 colonies results in a statistically inaccurate estimation of cell density. CFU should be between 30 and 300. <br><sup>2</sup> Colonies formed in one quadrant (88) were counted and multiplied by 4 to give final cell density.<br> <sup>3</sup> Positive control was plated on plain LB plates, without amp. | ||
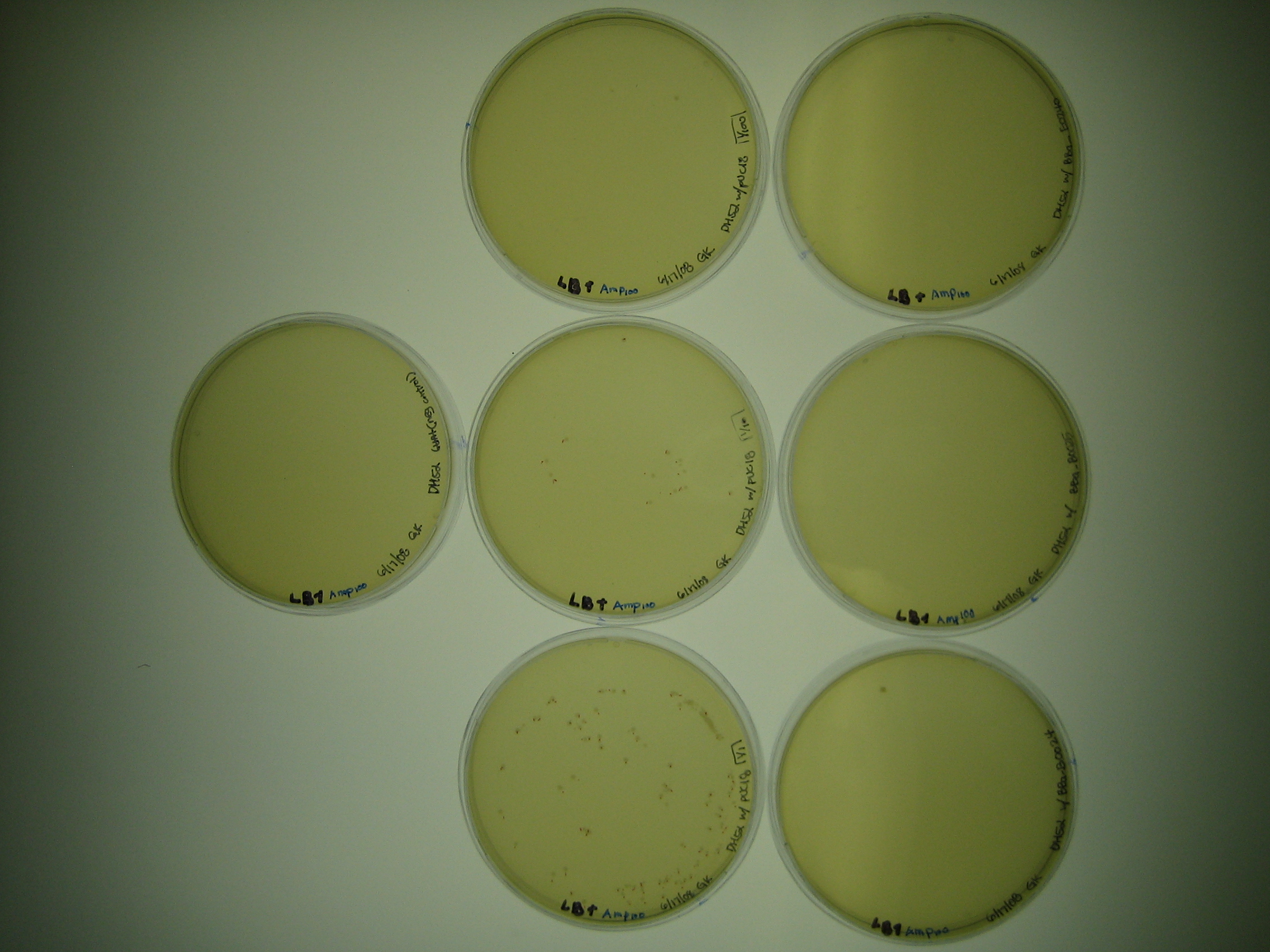
| - | + | [[Image:DSC00701.JPG| left|thumb|240 px |DH5-α transformants Batch 1 and 2: <br> | |
| + | ''(L-R)'' <br> | ||
| + | Row 1: Callahan cells 1/1, 1/10, 1/100 <br> | ||
| + | Row 2: 4-18 cells 1/1, 1/10, 1/100 <br> | ||
| + | Row 3: 2-17 cells 1/1, 1/10, 1/100 <br> | ||
| + | Row 4: 4-17 and 2-15 cells positive and negative controls]] | ||
| + | |||
| + | |||
| + | [[Image:Competent_cell_batch3_test.jpg|left|thumb|240px|Batch 3]] | ||
| + | |||
| + | |||
| + | == Discussion == | ||
* Next time, remember to aliquot out and transform only 50 ul of the competent cells. | * Next time, remember to aliquot out and transform only 50 ul of the competent cells. | ||
* Number of transformants do not appear to differ between cells grown to OD600=0.3 and OD600=0.6 prior to induction of chemical competency | * Number of transformants do not appear to differ between cells grown to OD600=0.3 and OD600=0.6 prior to induction of chemical competency | ||
* Callahan lab cells appear 10 times more competent. Did he start with more competent cells (higher cell density)? Does exponential growth of E. coli seed stocks at 23C (Callahan cells, recommended protocol) result in higher competency than growth of E. coli at 37C (our cells)? | * Callahan lab cells appear 10 times more competent. Did he start with more competent cells (higher cell density)? Does exponential growth of E. coli seed stocks at 23C (Callahan cells, recommended protocol) result in higher competency than growth of E. coli at 37C (our cells)? | ||
| + | = Test Competent DB3.1 Cells = | ||
| + | :#These cells were made on 7/11/08 with this [[Team:Hawaii/Make Competent E. Coli|protocol]]. | ||
{{Team:Hawaii/Footer}} | {{Team:Hawaii/Footer}} | ||
| + | |||
| + | :#Competency was tested during a transformation from [[Team:Hawaii/Notebook/2008-07-14|7/14/08]]. | ||
| + | |||
| + | <strong>Procedure</strong> | ||
| + | # Thaw plasmid on ice | ||
| + | # Add 2uL plasmid pUC18 | ||
| + | # Incubate plasmid + competent cells on ice for 10 minutes | ||
| + | # Heat shock 60 sec at 42C | ||
| + | # Add 200 μl SOC | ||
| + | # Incubate at 37 C for 2 hour in 2 ml eppendorf tubes, with shaking (235 rpm) | ||
| + | # Plate 250 uL dilutions on LB media | ||
| + | |||
| + | <strong>Results</strong> | ||
| + | |||
| + | :# The pUC18 transformation yielded a lawn while the (-) control yielded no colonies, verifying that DB3.1 competent cells can take up foreign DNA. | ||
| + | |||
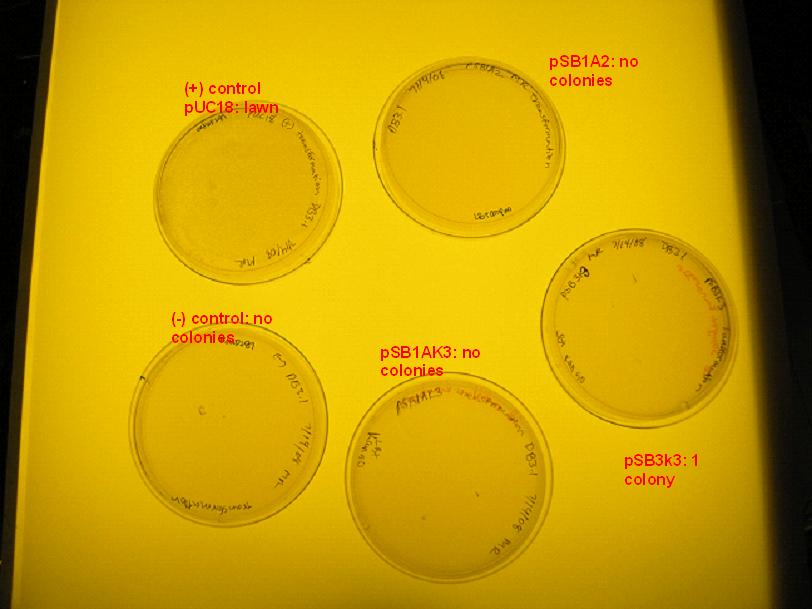
| + | [[Image:transformation_7_14_08.jpg|right|thumb|300px|The day after transformation. (+) control pUC18 yielded a lawn, the (-) control yielded no colonies. The results of the other transformations are included else where.]] | ||
Latest revision as of 01:33, 16 July 2008
Contents |
Test Competent E. coli DH5a
- Test our competent cells against Callahan competent cells
- Competency was chemically induced by RbCl
Methods
Materials
- Transported 50uL of competent cells from Callahan Lab on ice
- 100uL batch 1 competent cells made at OD600 ~0.6 (split into two 50 uL aliquots 4-18a,4-18b)
- 100uL batch 2 competent cells made at OD600 ~0.3 (split into two 50 uL aliquots 2-17a,2-17b)
Procedure
- Thaw plasmid on ice
- Add 1uL plasmid pBluescript
- Incubate plasmid + competent cells on ice for 30 minutes
- Heat shock 60 sec at 42C
- Add 250 μl SOC
- Incubate at 37 C for 1 hour in 2 ml eppendorf tubes, with shaking (150 rpm)
- Plate 20 uL dilutions 1/1, 1/10, 1/100 on LB+amp to test competency
Results
| Source DH5-α | Dilution | Colony Forming Units on Plate | Number of Transformed Cells per mL |
|---|---|---|---|
| Callahan lab | 1:1 | Too many to count | n/a |
| 1:10 | Too many to count | n/a | |
| 1:100 | 201 | 1,005,000 | |
| 2-17 (OD600=0.3) | 1:1 | Too many to count | n/a |
| 1:10 | 334 | 167,000 | |
| 1:100 | 17 [1] | 85,000 | |
| 4-18 (OD600=0.6) | 1:1 | Too many to count | n/a |
| 1:10 | 352 [2] | 176,000 | |
| 1:100 | 40 | 200,000 | |
| 4-17 (OD600=0.6) | 1:1 (negative control) | 0 | 0 |
| 2-15 (OD600=0.3) | 0 | 0 | |
| 4-17 (OD600=0.6) | 1:1 (positive control [3]) | Bacterial lawn | n/a |
| 2-15 (OD600=0.3) | Bacterial lawn | n/a |
Notes:
1 Fewer than 30 colonies results in a statistically inaccurate estimation of cell density. CFU should be between 30 and 300.
2 Colonies formed in one quadrant (88) were counted and multiplied by 4 to give final cell density.
3 Positive control was plated on plain LB plates, without amp.
Discussion
- Next time, remember to aliquot out and transform only 50 ul of the competent cells.
- Number of transformants do not appear to differ between cells grown to OD600=0.3 and OD600=0.6 prior to induction of chemical competency
- Callahan lab cells appear 10 times more competent. Did he start with more competent cells (higher cell density)? Does exponential growth of E. coli seed stocks at 23C (Callahan cells, recommended protocol) result in higher competency than growth of E. coli at 37C (our cells)?
Test Competent DB3.1 Cells
- These cells were made on 7/11/08 with this protocol.
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
- Competency was tested during a transformation from 7/14/08.
Procedure
- Thaw plasmid on ice
- Add 2uL plasmid pUC18
- Incubate plasmid + competent cells on ice for 10 minutes
- Heat shock 60 sec at 42C
- Add 200 μl SOC
- Incubate at 37 C for 2 hour in 2 ml eppendorf tubes, with shaking (235 rpm)
- Plate 250 uL dilutions on LB media
Results
- The pUC18 transformation yielded a lawn while the (-) control yielded no colonies, verifying that DB3.1 competent cells can take up foreign DNA.
 "
"