|
|
| (19 intermediate revisions not shown) |
| Line 3: |
Line 3: |
| | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Team|<font color="#ffffff">The Team</font>]] | | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Team|<font color="#ffffff">The Team</font>]] |
| | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Project|<font color="#ffffff">The Project</font>]] | | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Project|<font color="#ffffff">The Project</font>]] |
| - | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Parts|<font color="#ffffff">Parts Submitted to the Registry</font>]] | + | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Parts|<font color="#ffffff">Parts</font>]] |
| - | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Modeling|<font color="#ffffff">Modeling</font>]]
| + | |
| | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Notebook|<font color="#ffffff">Notebook</font>]] | | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Notebook|<font color="#ffffff">Notebook</font>]] |
| | + | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Modeling|<font color="#ffffff">Modeling</font>]] |
| | + | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Links|<font color="#ffffff">Links</font>]] |
| | + | !style="text-align:center; background-color:#cd0000; border-width:0px; padding:3px;"|[[Team:Montreal/Sponsors|<font color="#ffffff">Sponsors</font>]] |
| | |} | | |} |
| | | | |
| - | {| align="center" | + | |
| - | |align="center" |[[Image:Central_dogma.jpg]] | + | |
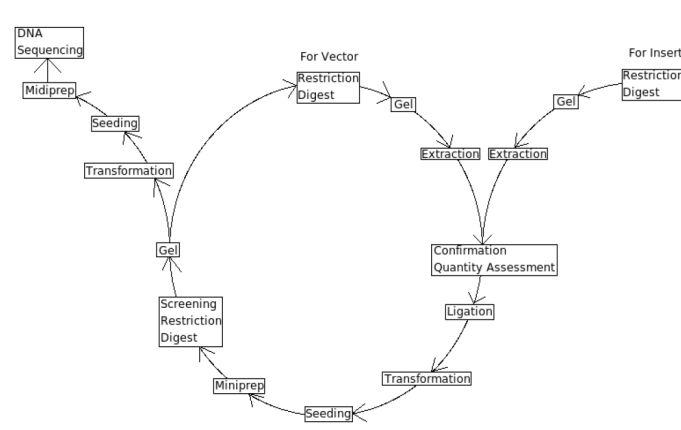
| | + | ==Lab Protocols== |
| | + | |
| | + | {| align="right" |
| | + | |align="right" |[[Image:Central_dogma.jpg]] |
| | |} | | |} |
| | | | |
| - | ==Lab Protocols==
| |
| | Key Elements of the Central Dogma of cloning | | Key Elements of the Central Dogma of cloning |
| | | | |
| Line 32: |
Line 37: |
| | | | |
| | ==Lab Progress== | | ==Lab Progress== |
| - | | + | <p><center> |
| - | '''[[Montreal/May|May 2008]]''' | '''[[Montreal/June|June 2008]]''' | '''[[Montreal/July|July 2008]]'''<br> | + | '''[[Team:Montreal/Notebook/May|May 2008]]''' | '''[[Team:Montreal/Notebook/June|June 2008]]''' | '''[[Team:Montreal/Notebook/July|July 2008]]''' | '''[[Team:Montreal/Notebook/August|August 2008]]''' | '''[[Team:Montreal/Notebook/September|September 2008]]''' | '''[[Team:Montreal/Notebook/October|October 2008]]''' | '''[[Team:Montreal/Notebook/November|November 2008]]'''</center><br> |
| - | | + | |
| - | <p>'''May 21st, 2008''': <ul>Restriction enzyme digest was done on the J-brick with EcoRI. Gel was run on the J-brick after the restriction digest. No DNA was detected on the gel (the ladder was visible on the gel).</ul></p>
| + | |
| - | | + | |
| - | <p>'''May 22nd, 2008''': <ul>Prepared TOP10 competent cells for eventual transformation.</ul>
| + | |
| - | <ul>Performed Mini-prep on Reporter+ Cells</ul>
| + | |
| - | <ul>Performed Digest and Gel on Reporter plasmid extract -- no DNA present, suggest follow-up maxi-prep</ul></p>
| + | |
| - | | + | |
| - | <p>'''May 23rd, 2008''': <ul>Transformed Top10 cells with Puc19 to ensure that the competent cell procedure was successful. Growth was observed, therefore procedure was successful.</ul></p>
| + | |
| - | | + | |
| - | <p>'''May 25th, 2008''': <ul>Diluted Reporter cells 1/1000 with 5ul Kan/mL culture for 16h incubation at 5:30pm. To be used for Maxiprep at 9:30am-1:30pm.</ul></p>
| + | |
| - | | + | |
| - | <p>'''May 28th, 2008''':<ul> Ran gel on Elowitz Reporter DNA cut with EcoR1; 2 bands</ul>
| + | |
| - | <ul>0.7 kb and 2.0 kb, confirms identity of reporter DNA.</ul>
| + | |
| - | <ul>Seeded syn-I and J-40001 into amp/kan LB and kan LB.</ul></p>
| + | |
| - | | + | |
| - | <p>'''May 29th, 2008''': <ul>Growth of J-brick in culture - No growth of I-brick on culture</ul>
| + | |
| - | <ul> Seeded J-brick for Midi-Prep in 40mL LB with ampicillin</ul>
| + | |
| - | <ul>Transformed TOP10 cells with both I brick and Reporter Plasmid</ul></p>
| + | |
| - | | + | |
| - | <p>'''June 2nd, 2008''':<ul>Growth of I-brick on culture</ul>
| + | |
| - | <ul>Midi-prep of both I and J brick followed by gel</ul>
| + | |
| - | <ul>Gel indicates no presence of DNA, will be confirmed by spectrophotometric assay</ul></p>
| + | |
| - | | + | |
| - | <p>'''June 3rd, 2008''':
| + | |
| - | <ul>Seeding of 5mL cultures of both I and J brick</ul>
| + | |
| - | <ul>Identity of colonies on I brick plates is suspect, must ensure that eventual DNA gel confirms exact restriction digest</ul></p> | + | |
| - | | + | |
| - | <p>'''June 4th, 2008''':
| + | |
| - | <ul>Midiprep was done on the I and the J-brick. Once the isopropanol was added, the J-brick midiprep looked clear (no DNA was eluted). DNA gel needs to be done to confirm presence of DNA in both cases.</ul></p>
| + | |
| - | | + | |
| - | <p>'''June 9th, 2008''':
| + | |
| - | <ul>Seeded J and I-brick re-seeded for Maxi-Prep</ul>
| + | |
| - | <ul>Gel failed to confirm presence of previously collected DNA samples of J and I-brick, will be repeated following Maxi-Prep.</ul></p>
| + | |
| - | | + | |
| - | <p>'''June 10th, 2008''':
| + | |
| - | <ul>Since no growth was observed in the I-brick culture, the I brick was re-seeded.</ul>
| + | |
| - | <ul>The J-brick was diluted in 500-ml of LB broth(for a Maxiprep to be done the next day).</ul></p>
| + | |
| - | | + | |
| - | <p>'''June 11th, 2008''':<ul> Maxiprep of the J-brick.</ul>
| + | |
| - | <ul>Restriction digest of J-brick.</ul>
| + | |
| - | <ul>Dilution of I-brick in 500-ml of LB broth. </ul></p>
| + | |
| - | | + | |
| - | <p>'''June 12th, 2008''':<ul> Maxiprep of the I-brick.</ul>
| + | |
| - | <ul>Restriction digest and gel of June 11th and June 12th I-brick and J-brick DNA using EcoR1. Bands revealed at roughly 4000bp and 2500bp for I-brick (expected 2652bp and 3939bp). J brick single band that was not informative, new digestion to be completed tomorrow.</ul></p>
| + | |
| - | | + | |
| - | <p>'''June 16th, 2008''':<ul> I-brick was seeded and diluted over last two days, but there was insufficient growth so it will be left to grow one more day before performing another midi-prep. This is to compliment the already successful Maxi-Prep that gave low concentrations of DNA.</ul>
| + | |
| - | <ul>Another gel was performed of previous J-brick preps that confirmed the absence of the desired plasmid, no DNA was detected when digested with EcoR1.</ul>
| + | |
| - | <ul>J-brick was re-transformed into TOP10 chemically competent cells and then plated on Amp+ plates.<ul/></p>
| + | |
 "
"