Team:University of Lethbridge/Notebook/GeneralLabJune
From 2008.igem.org
Nathan.puhl (Talk | contribs) |
Munima.alam (Talk | contribs) m |
||
| (16 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Team:University_of_Lethbridge/Notebook|Back to The University of Lethbridge Main Notebook]] | ||
| + | |||
===June 6 2008=== | ===June 6 2008=== | ||
| - | ====Sebastian and Roxanne ==== | + | ====Sebastian, John and Roxanne ==== |
Prepared 1L of semi-solid media following the procedure found on OpenWetWare. | Prepared 1L of semi-solid media following the procedure found on OpenWetWare. | ||
-10g peptone (substituted for tryptone) | -10g peptone (substituted for tryptone) | ||
| Line 8: | Line 10: | ||
Stored media in fridge. | Stored media in fridge. | ||
| + | |||
===June 10 2008=== | ===June 10 2008=== | ||
====Christa, Munima, Roxanne, and Sebastian==== | ====Christa, Munima, Roxanne, and Sebastian==== | ||
Prepared 1L of liquid media following the procedure found on OpenWetWare. | Prepared 1L of liquid media following the procedure found on OpenWetWare. | ||
| - | -10g peptone | + | -10g peptone (substituted for tryptone) |
-10g NaCl | -10g NaCl | ||
-5g Yeast Extract | -5g Yeast Extract | ||
Poured 36 culture test tubes, and the remainder was left in a 1L Erlenmeyer flask. Stored both in fridge. | Poured 36 culture test tubes, and the remainder was left in a 1L Erlenmeyer flask. Stored both in fridge. | ||
| - | |||
====Christa==== | ====Christa==== | ||
| Line 25: | Line 27: | ||
====Roxanne==== | ====Roxanne==== | ||
Defrosted the -20 freezer in the teaching lab for iGEM use (with a little help from my friends!) | Defrosted the -20 freezer in the teaching lab for iGEM use (with a little help from my friends!) | ||
| + | |||
===June 11 2008=== | ===June 11 2008=== | ||
====Sebastian, Munima, Roxanne, Christa==== | ====Sebastian, Munima, Roxanne, Christa==== | ||
| - | Poured 8 minimal media (labeled control- with blue sharpie) plates and 14 amp plates (labeled amp - with red sharpie) stored in the 4 C fridge. Amp concentration is always 50ug/mL. | + | Poured 8 minimal media (labeled control - with blue sharpie) plates and 14 amp plates (labeled amp - with red sharpie) |
| + | stored in the 4 C fridge. Amp concentration is always 50ug/mL. | ||
| + | |||
===June 16 2008=== | ===June 16 2008=== | ||
====Nathan Puhl, Munima, Christa, Sebastian, Roxanne==== | ====Nathan Puhl, Munima, Christa, Sebastian, Roxanne==== | ||
| - | + | Transformed supercompetent cells with basic biobrick vector [http://partsregistry.org/wiki/index.php?title=Part:pSB1A7 pSB1A7] (ampicillin resistance). | |
| - | Transformed | + | -50 uL of DH5-alpha cells, 0.85 uL 2-mercaptoethanol, 1 uL of plasmid dissolved with 15 uL of ddH2O |
| - | -50 uL of cells, 0.85 uL 2-mercaptoethanol, 1 uL of plasmid dissolved with 15 uL of ddH2O | + | |
-30 min on ice | -30 min on ice | ||
-45 s at 42 C | -45 s at 42 C | ||
-2 min on ice | -2 min on ice | ||
-Add 0.9 mL of LB shaker incubate at 225 RPM and 37 C | -Add 0.9 mL of LB shaker incubate at 225 RPM and 37 C | ||
| + | |||
===June 17 2008=== | ===June 17 2008=== | ||
====Munima, Christa, Nathan Puhl==== | ====Munima, Christa, Nathan Puhl==== | ||
Checked plates | Checked plates | ||
| - | + | -Only one colony from transformation, not very good efficiency, don't know why because too many possibilities, most | |
| - | -Only one colony from transformation, not very good efficiency, don't know why because too many | + | likely amount of DNA due to inability to quantify plasmid from iGEM plates |
-Subcultured colony in liquid LB + amp | -Subcultured colony in liquid LB + amp | ||
-Plate 200 uL on LB + amp at 37 C overnight | -Plate 200 uL on LB + amp at 37 C overnight | ||
| + | |||
| + | |||
| + | ===June 18 2008=== | ||
| + | ====Munima, Christa, Alix, Nathan Puhl==== | ||
| + | Made glycerol stock of pSB1A7 transformed E. coli | ||
| + | |||
| + | Extracted plasmid from transformed E. coli using the Eppendorf FastPlasmid minikit and stored 4 aliquots of 25 uL in the -20 C freezer. | ||
| + | |||
| + | |||
| + | ===June 19 2008=== | ||
| + | ====Nathan Puhl, Alix, Munima, Christa, Roxanne==== | ||
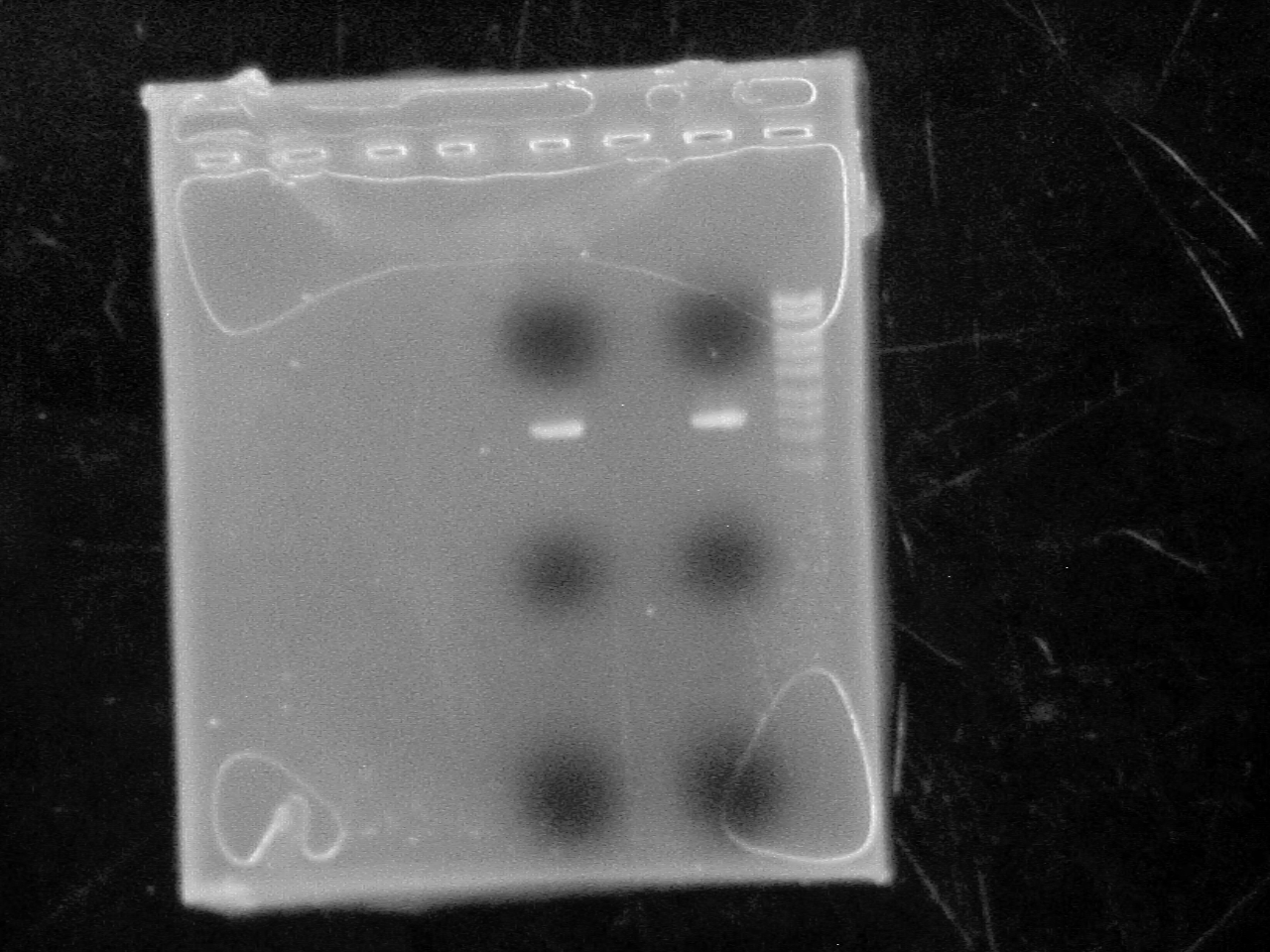
| + | Ran plasmids on 1% agarose gel with High range ladder | ||
| + | |||
| + | [[Image:pSB1A7 plasmid.jpg|500 px]] | ||
| + | |||
| + | plasmid is ~15 ng/uL | ||
| + | |||
| + | |||
| + | ===June 24 2008=== | ||
| + | ====Nathan Puhl, Alix==== | ||
| + | Streaked [http://partsregistry.org/wiki/index.php?title=Part:BBa_I13522 BBa_I13522] (TetR repressed GFP) onto LB + amp from last year's glycerol stock. | ||
| + | |||
| + | |||
| + | ===June 25, 2008=== | ||
| + | ====Nathan Puhl, Sebastian, Alix==== | ||
| + | Subcultured BBa_I13522 into liquid LB + amp for plasmid mini prep | ||
| + | |||
| + | |||
| + | ===June 26, 2008=== | ||
| + | ====Nathan Puhl, Sebastian, Alix==== | ||
| + | Transformed [http://partsregistry.org/wiki/index.php?title=Part:BBa_J5526 BBa_J5526] (RFP complete), [http://partsregistry.org/wiki/index.php?title=Part:BBa_I730002 BBa_I730002] (pLACI-|TetR), and [http://partsregistry.org/wiki/index.php?title=Part:BBa_B0015 BBa_B0015] (Double T). | ||
| + | |||
| + | Plasmid mini-prepped GFP complete (BBa_I13522). | ||
| + | |||
| + | |||
| + | ===June 27, 2008=== | ||
| + | ====Nathan Puhl, Alix, Munima==== | ||
| + | No colonies on any plates. Will try again next week. | ||
Latest revision as of 16:31, 25 August 2008
Back to The University of Lethbridge Main Notebook
Contents |
June 6 2008
Sebastian, John and Roxanne
Prepared 1L of semi-solid media following the procedure found on OpenWetWare.
-10g peptone (substituted for tryptone) -10g Agar -10g NaCl -5g Yeast Extract
Stored media in fridge.
June 10 2008
Christa, Munima, Roxanne, and Sebastian
Prepared 1L of liquid media following the procedure found on OpenWetWare.
-10g peptone (substituted for tryptone) -10g NaCl -5g Yeast Extract
Poured 36 culture test tubes, and the remainder was left in a 1L Erlenmeyer flask. Stored both in fridge.
Christa
Made an inventory of iGEM 2007 parts in Wieden -80 freezer inventory.xls
Roxanne
Defrosted the -20 freezer in the teaching lab for iGEM use (with a little help from my friends!)
June 11 2008
Sebastian, Munima, Roxanne, Christa
Poured 8 minimal media (labeled control - with blue sharpie) plates and 14 amp plates (labeled amp - with red sharpie) stored in the 4 C fridge. Amp concentration is always 50ug/mL.
June 16 2008
Nathan Puhl, Munima, Christa, Sebastian, Roxanne
Transformed supercompetent cells with basic biobrick vector [http://partsregistry.org/wiki/index.php?title=Part:pSB1A7 pSB1A7] (ampicillin resistance).
-50 uL of DH5-alpha cells, 0.85 uL 2-mercaptoethanol, 1 uL of plasmid dissolved with 15 uL of ddH2O -30 min on ice -45 s at 42 C -2 min on ice -Add 0.9 mL of LB shaker incubate at 225 RPM and 37 C
June 17 2008
Munima, Christa, Nathan Puhl
Checked plates
-Only one colony from transformation, not very good efficiency, don't know why because too many possibilities, most likely amount of DNA due to inability to quantify plasmid from iGEM plates -Subcultured colony in liquid LB + amp -Plate 200 uL on LB + amp at 37 C overnight
June 18 2008
Munima, Christa, Alix, Nathan Puhl
Made glycerol stock of pSB1A7 transformed E. coli
Extracted plasmid from transformed E. coli using the Eppendorf FastPlasmid minikit and stored 4 aliquots of 25 uL in the -20 C freezer.
June 19 2008
Nathan Puhl, Alix, Munima, Christa, Roxanne
Ran plasmids on 1% agarose gel with High range ladder
plasmid is ~15 ng/uL
June 24 2008
Nathan Puhl, Alix
Streaked [http://partsregistry.org/wiki/index.php?title=Part:BBa_I13522 BBa_I13522] (TetR repressed GFP) onto LB + amp from last year's glycerol stock.
June 25, 2008
Nathan Puhl, Sebastian, Alix
Subcultured BBa_I13522 into liquid LB + amp for plasmid mini prep
June 26, 2008
Nathan Puhl, Sebastian, Alix
Transformed [http://partsregistry.org/wiki/index.php?title=Part:BBa_J5526 BBa_J5526] (RFP complete), [http://partsregistry.org/wiki/index.php?title=Part:BBa_I730002 BBa_I730002] (pLACI-|TetR), and [http://partsregistry.org/wiki/index.php?title=Part:BBa_B0015 BBa_B0015] (Double T).
Plasmid mini-prepped GFP complete (BBa_I13522).
June 27, 2008
Nathan Puhl, Alix, Munima
No colonies on any plates. Will try again next week.
 "
"