Team:Tokyo Tech/Development of low pressure inducible promoter
From 2008.igem.org
(→Development of low pressure inducible promoter) |
(→Strategy) |
||
| (6 intermediate revisions not shown) | |||
| Line 18: | Line 18: | ||
== <font size="5">Development of low pressure inducible promoter </font>== | == <font size="5">Development of low pressure inducible promoter </font>== | ||
<html> | <html> | ||
| - | <body> | + | <body> |
| - | + | <div> | |
| - | <div>It is known that lac promoter is induced under 30 MPa (Kato et al. 1994). | + | It is known that lac promoter is induced under 30 MPa (Kato et al. 1994).However, 30 MPa is too high to use as input. |
| - | However, 30 MPa is too high to use as input.</div> | + | </div> |
| - | <div>Therefore, we are trying to develop low pressure inducible promoter.</div> | + | <div> |
| - | </body> | + | Therefore, we are trying to develop low pressure inducible promoter. |
| + | </div> | ||
| + | </body> | ||
</html> | </html> | ||
| Line 33: | Line 35: | ||
=== Strategy === | === Strategy === | ||
| - | <gallery widths=" | + | <gallery widths="360px" heights="190px" > |
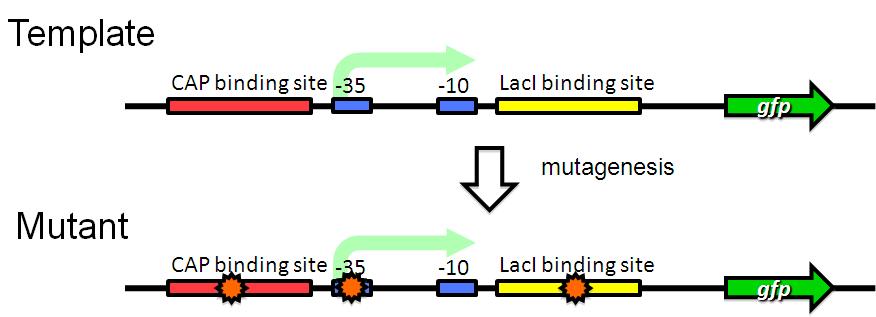
| - | Image:Tech dev 1.JPG|Figure 1 - RCP random mutagenesis | + | Image:Tech dev 1.JPG|Figure 1 - RCP random mutagenesis |
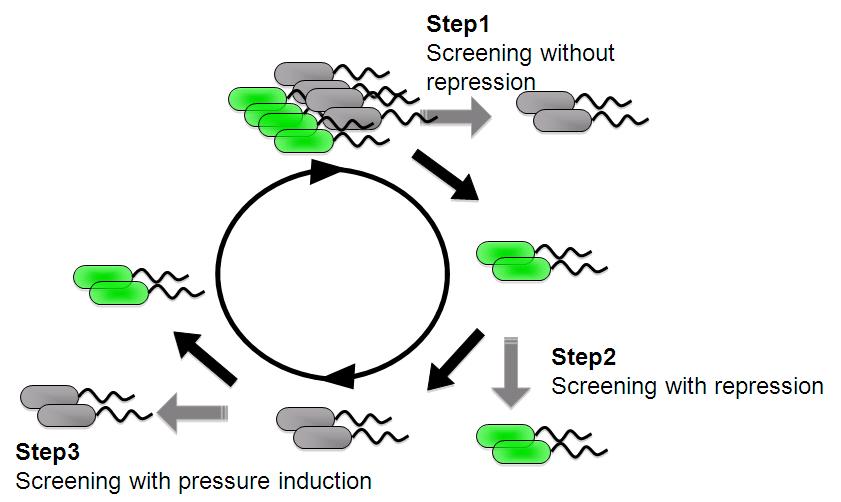
| - | Image:Tech dev 2.JPG|Figure 2 - Strategy of sorting with FACS | + | Image:Tech dev 2.JPG|Figure 2 - Strategy of sorting with FACS |
</gallery> | </gallery> | ||
| Line 41: | Line 43: | ||
And we are screening an E. coli library for promoters that are induced under low pressure using a fluorescence activated cell sorter (FACS). | And we are screening an E. coli library for promoters that are induced under low pressure using a fluorescence activated cell sorter (FACS). | ||
This scheme is based on the ability to separate bacteria with a FACS in response to expression, or lack of expression, of a fluorescent marker. | This scheme is based on the ability to separate bacteria with a FACS in response to expression, or lack of expression, of a fluorescent marker. | ||
| - | + | *'''step 1''' - Fluorescent bacteria without repressor protein were collected by FACS. This sorted pool contains bacteria bearing both constitutive and low pressure-inducible promoter. | |
| - | Fluorescent bacteria without repressor protein were collected by FACS. This sorted pool contains bacteria bearing both constitutive and low pressure-inducible promoter. | + | |
| - | + | *'''step 2''' - Constitutive promoter are removed with repressor protein and sorting all non-fluorescent bacteria. | |
| - | Constitutive promoter are removed with repressor protein and sorting all non-fluorescent bacteria. | + | |
| - | + | *'''step 3''' - A final passage through under pressure with repressor protain and sorting for fluorescent bacteria removes false negatives and enriches for bacteria bearing promoter that are low pressure inducible. | |
| - | A final passage through under pressure with repressor protain and sorting for fluorescent bacteria removes false negatives and enriches for bacteria bearing promoter that are low pressure inducible. | + | |
== Results== | == Results== | ||
| Line 52: | Line 53: | ||
Fluorescent and non-fluorescent bacteria were sorted and we characterized their promoter. | Fluorescent and non-fluorescent bacteria were sorted and we characterized their promoter. | ||
=== Sequence and Characterization=== | === Sequence and Characterization=== | ||
| - | <gallery widths=" | + | <gallery widths="430px" heights="220px" > |
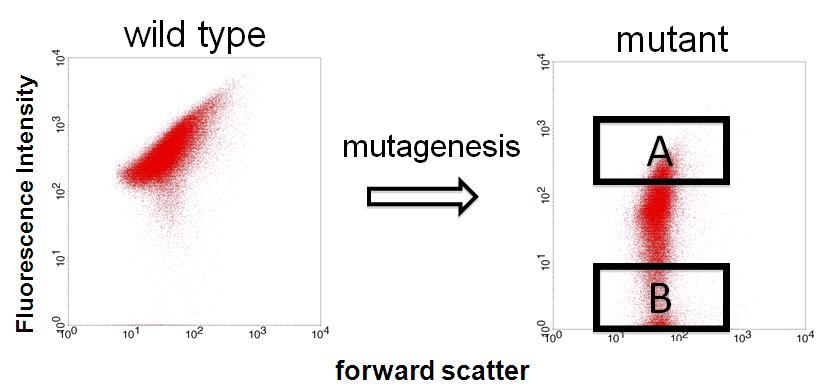
| - | Image:Dev 3.JPG|Figure 3 - FACS | + | Image:Dev 3.JPG|Figure 3 - FACS |
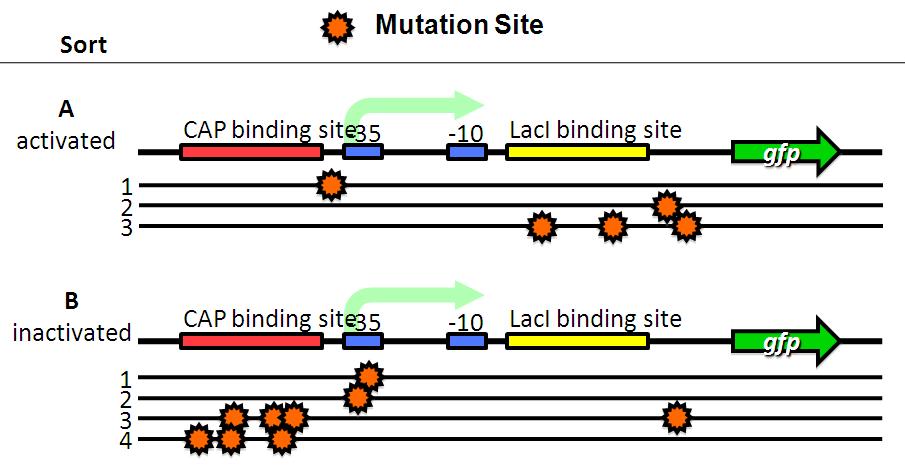
| - | Image:Tech dev 4.JPG|Figure 4 - Sequensing | + | Image:Tech dev 4.JPG|Figure 4 - Sequensing |
</gallery> | </gallery> | ||
We sorted fluorescent (A) and non-fluorescent bacteria (B) with a FACS. | We sorted fluorescent (A) and non-fluorescent bacteria (B) with a FACS. | ||
Then, we analyze these base sequences. | Then, we analyze these base sequences. | ||
| - | A have mutations in lacI binding site or non-functional DNA. | + | *A have mutations in lacI binding site or non-functional DNA. |
| - | B have mutations in CAP binding site, -35 or non-functional DNA. | + | *B have mutations in CAP binding site, -35 or non-functional DNA. |
== Conclusion== | == Conclusion== | ||
We have successfully demonstrated that it is possible to collect objective promoter by PCR random mutagenesis and screening with a FACS. | We have successfully demonstrated that it is possible to collect objective promoter by PCR random mutagenesis and screening with a FACS. | ||
So we clearly believe that we can screen low pressure inducible lac promoter mutant with this strategy. | So we clearly believe that we can screen low pressure inducible lac promoter mutant with this strategy. | ||
Latest revision as of 11:01, 26 October 2008
| Home | Construction | Acrylic container | Development of promoter | Genetic toggle switch | Parts Submitted to the Registry | Result | The Team | Acknowledgements |
|---|
Contents |
Development of low pressure inducible promoter
Method
Principle
LacI binds to lacI binding site and repress lac promoter. In addition, pressure of 30 MPa activated the lac promoter. We propose a hypothesis that 30 MPa induce a conformational change in lacI reprssure and change the affinity for lacI binding site weaker. Therefore, lacI can't repress lac promoter under 30 MPa.
Strategy
We are trying to develop low pressure inducible promoter by PCR random mutagenesis to lac promoter in order to reduce affitity to lacI. And we are screening an E. coli library for promoters that are induced under low pressure using a fluorescence activated cell sorter (FACS). This scheme is based on the ability to separate bacteria with a FACS in response to expression, or lack of expression, of a fluorescent marker.
- step 1 - Fluorescent bacteria without repressor protein were collected by FACS. This sorted pool contains bacteria bearing both constitutive and low pressure-inducible promoter.
- step 2 - Constitutive promoter are removed with repressor protein and sorting all non-fluorescent bacteria.
- step 3 - A final passage through under pressure with repressor protain and sorting for fluorescent bacteria removes false negatives and enriches for bacteria bearing promoter that are low pressure inducible.
Results
We finished step 1. Fluorescent and non-fluorescent bacteria were sorted and we characterized their promoter.
Sequence and Characterization
We sorted fluorescent (A) and non-fluorescent bacteria (B) with a FACS. Then, we analyze these base sequences.
- A have mutations in lacI binding site or non-functional DNA.
- B have mutations in CAP binding site, -35 or non-functional DNA.
Conclusion
We have successfully demonstrated that it is possible to collect objective promoter by PCR random mutagenesis and screening with a FACS. So we clearly believe that we can screen low pressure inducible lac promoter mutant with this strategy.
 "
"