TUDelft/17 September 2008
From 2008.igem.org
(New page: {{Template:TUDelftiGEM2008}} {{Template:TUDelftiGEM2008_menu_home}} {{Template:TUDelftiGEM2008_calendar}} =September 17th 2008= ==Miniprep== We did a miniprep on the culture of [http://p...) |
m |
||
| (5 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
{{Template:TUDelftiGEM2008_calendar}} | {{Template:TUDelftiGEM2008_calendar}} | ||
| - | =September 17th | + | =September 17th= |
==Miniprep== | ==Miniprep== | ||
| Line 13: | Line 13: | ||
==Gel analysis of previous miniprep== | ==Gel analysis of previous miniprep== | ||
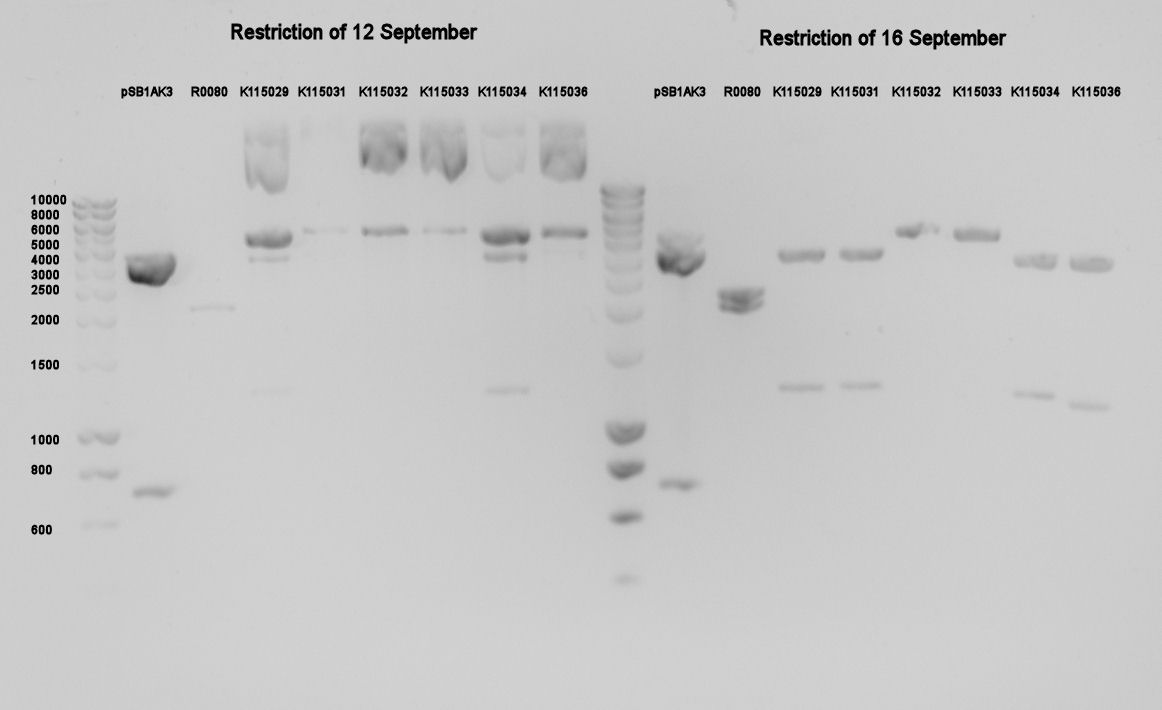
Due to the failing of the transformation yesterday, we wanted to put the cut and purified DNA on gel. This can be seen in figure 1. | Due to the failing of the transformation yesterday, we wanted to put the cut and purified DNA on gel. This can be seen in figure 1. | ||
| + | [[Image:TUDelft170908restrict.jpg|thumb|center|Figure 1. Restriction of the previous 3A-assemblies. A marker has been loaded twice.]] | ||
| + | |||
| + | It can be seen the restrictions of 12 September are far from complete, which also indicates why the ligations/transformations didn't work. The restrictions of 16 September are better, but next time we will cut quantities of over 1000 ng (pSB1AT3 & R0080) vector with 1 ul per restriction enzyme. Furthermore K115032 and K115033 look incompletely cut, so we won't expect very good transformations of these parts. These two parts are however highly similar to K115029 and K115030, so these two constructs are the least important ones to measure. | ||
==Growing for luciferase assay== | ==Growing for luciferase assay== | ||
| - | + | As we have the control and a thermosensitive part, we'll start growing cultures today to do an assay on Friday. We want to test what will be the fastest way for obtaining samples, therefore we started a range of cultures, both growing conditions are on K115034 (designed to switch @ 27ºC) and K115012 (reference). | |
| - | + | All cultures grown were shaken with a 1 inch stroke at 175 rpm. | |
| + | * Growing overnight with arabinose induction at room temperature | ||
| + | * Growing overnight with arabinose induction at 37ºC | ||
| + | * Growing overnight without induction at 37ºC (to produce biomass), induce next day for 5 hours at room temperature (to produce luciferase) | ||
| + | * Growing 2 times overnight (ca. 36 h) with arabinose induction at room temperature | ||
| + | * There was one extra sample of K115012 to test R0080 for leakiness. This sample was grown o/n at 37ºC without arabinose induction. | ||
{{Template:TUDelftiGEM2008_sidebar}} | {{Template:TUDelftiGEM2008_sidebar}} | ||
Latest revision as of 11:18, 27 October 2008
| July | ||||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| 28 | 29 | 30 | 31 | |||
| August | ||||||
| M | T | W | T | F | S | S |
| 1 | 2 | 3 | ||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 25 | 26 | 27 | 28 | 29 | 30 | 31 |
| September | ||||||
| M | T | W | T | F | S | S |
| [http://2008.igem.org/TUDelft/1_September_2008 1] | [http://2008.igem.org/TUDelft/2_September_2008 2] | [http://2008.igem.org/TUDelft/3_September_2008 3] | [http://2008.igem.org/TUDelft/4_September_2008 4] | [http://2008.igem.org/TUDelft/5_September_2008 5] | [http://2008.igem.org/wiki/index.php?title=TUDelft/6_September_2008&action=edit 6] | [http://2008.igem.org/wiki/index.php?title=TUDelft/7_September_2008&action=edit 7] |
| [http://2008.igem.org/TUDelft/8_September_2008 8] | [http://2008.igem.org/TUDelft/9_September_2008 9] | [http://2008.igem.org/TUDelft/10_September_2008 10] | [http://2008.igem.org/TUDelft/11_September_2008 11] | [http://2008.igem.org/TUDelft/12_September_2008 12] | [http://2008.igem.org/wiki/index.php?title=TUDelft/13_September_2008&action=edit 13] | [http://2008.igem.org/wiki/index.php?title=TUDelft/14_September_2008&action=edit 14] |
| [http://2008.igem.org/TUDelft/15_September_2008 15] | [http://2008.igem.org/TUDelft/16_September_2008 16] | [http://2008.igem.org/TUDelft/17_September_2008 17] | [http://2008.igem.org/TUDelft/18_September_2008 18] | [http://2008.igem.org/TUDelft/19_September_2008 19] | [http://2008.igem.org/wiki/index.php?title=TUDelft/20_September_2008&action=edit 20] | [http://2008.igem.org/wiki/index.php?title=TUDelft/21_September_2008&action=edit 21] |
| [http://2008.igem.org/TUDelft/22_September_2008 22] | [http://2008.igem.org/TUDelft/23_September_2008 23] | [http://2008.igem.org/TUDelft/24_September_2008 24] | [http://2008.igem.org/TUDelft/25_September_2008 25] | [http://2008.igem.org/wiki/index.php?title=TUDelft/26_September_2008&action=edit 26] | [http://2008.igem.org/wiki/index.php?title=TUDelft/27_September_2008&action=edit 27] | [http://2008.igem.org/wiki/index.php?title=TUDelft/28_September_2008&action=edit 28] |
| [http://2008.igem.org/TUDelft/29_September_2008 29] | [http://2008.igem.org/TUDelft/30_September_2008 30] | |||||
| October | ||||||
| M | T | W | T | F | S | S |
| [http://2008.igem.org/TUDelft/1_October_2008 1] | [http://2008.igem.org/TUDelft/2_October_2008 2] | [http://2008.igem.org/TUDelft/3_October_2008 3] | [http://2008.igem.org/wiki/index.php?title=TUDelft/4_October_2008&action=edit 4] | [http://2008.igem.org/wiki/index.php?title=TUDelft/5_October_2008&action=edit 5] | ||
| [http://2008.igem.org/TUDelft/6_October_2008 6] | [http://2008.igem.org/TUDelft/7_October_2008 7] | [http://2008.igem.org/TUDelft/8_October_2008 8] | [http://2008.igem.org/TUDelft/9_October_2008 9] | [http://2008.igem.org/TUDelft/10_October_2008 10] | [http://2008.igem.org/wiki/index.php?title=TUDelft/11_October_2008&action=edit 11] | [http://2008.igem.org/wiki/index.php?title=TUDelft/12_October_2008&action=edit 12] |
| [http://2008.igem.org/TUDelft/13_October_2008 13] | [http://2008.igem.org/TUDelft/14_October_2008 14] | [http://2008.igem.org/TUDelft/15_October_2008 15] | [http://2008.igem.org/TUDelft/16_October_2008 16] | [http://2008.igem.org/TUDelft/17_October_2008 17] | [http://2008.igem.org/wiki/index.php?title=TUDelft/18_October_2008&action=edit 18] | [http://2008.igem.org/wiki/index.php?title=TUDelft/19_October_2008&action=edit 19] |
| [http://2008.igem.org/TUDelft/20_October_2008 20] | [http://2008.igem.org/TUDelft/21_October_2008 21] | [http://2008.igem.org/TUDelft/22_October_2008 22] | [http://2008.igem.org/TUDelft/23_October_2008 23] | [http://2008.igem.org/TUDelft/24_October_2008 24] | [http://2008.igem.org/wiki/index.php?title=TUDelft/25_October_2008&action=edit 25] | [http://2008.igem.org/wiki/index.php?title=TUDelft/26_October_2008&action=edit 26] |
| [http://2008.igem.org/wiki/index.php?title=TUDelft/27_October_2008&action=edit 27] | [http://2008.igem.org/wiki/index.php?title=TUDelft/28_October_2008&action=edit 28] | [http://2008.igem.org/wiki/index.php?title=TUDelft/29_October_2008&action=edit 29] | [http://2008.igem.org/wiki/index.php?title=TUDelft/30_October_2008&action=edit 30] | [http://2008.igem.org/wiki/index.php?title=TUDelft/31_October_2008&action=edit 31] | ||
Contents |
September 17th
Miniprep
We did a miniprep on the culture of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K115034 K115034], possibly we will try to have it sequenced, although it's a bit long. The end concentration was ca. 300 ng/ul in 100 ul.
Transformation
Due to failing of the previous transformations of our end RNA thermometer testing parts, we've started new ligations, which we transformed today.
Gel analysis of previous miniprep
Due to the failing of the transformation yesterday, we wanted to put the cut and purified DNA on gel. This can be seen in figure 1.
It can be seen the restrictions of 12 September are far from complete, which also indicates why the ligations/transformations didn't work. The restrictions of 16 September are better, but next time we will cut quantities of over 1000 ng (pSB1AT3 & R0080) vector with 1 ul per restriction enzyme. Furthermore K115032 and K115033 look incompletely cut, so we won't expect very good transformations of these parts. These two parts are however highly similar to K115029 and K115030, so these two constructs are the least important ones to measure.
Growing for luciferase assay
As we have the control and a thermosensitive part, we'll start growing cultures today to do an assay on Friday. We want to test what will be the fastest way for obtaining samples, therefore we started a range of cultures, both growing conditions are on K115034 (designed to switch @ 27ºC) and K115012 (reference). All cultures grown were shaken with a 1 inch stroke at 175 rpm.
- Growing overnight with arabinose induction at room temperature
- Growing overnight with arabinose induction at 37ºC
- Growing overnight without induction at 37ºC (to produce biomass), induce next day for 5 hours at room temperature (to produce luciferase)
- Growing 2 times overnight (ca. 36 h) with arabinose induction at room temperature
- There was one extra sample of K115012 to test R0080 for leakiness. This sample was grown o/n at 37ºC without arabinose induction.
 "
"