|
B. subtilis can act as a cell factory producing and secreting proteins. Proteins can be secreted via three major pathways within the B. subtilis: Secretory signal recognition particle (Sec-SRP) pathway, Twin-arginine translocation (Tat) pathway and the ATP-binding cassette (ABC) transporters. The Sec-SRP pathway would be more suitable in this case because it has been well studied in comparison to the two latter pathways.
The process of Sec-SRP-dependent proteins secretion
- SRP-protein targeting system to cell membrane
- Sec protein translocation machinery across the cytoplasmic membrane
- Folding and release of protein
Signal Peptides
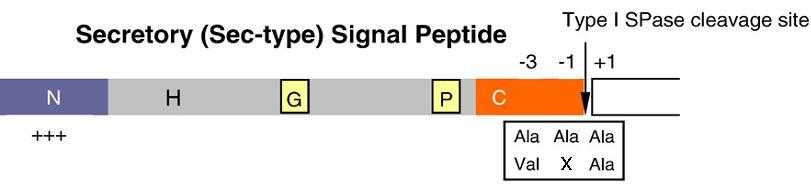
Signal peptides are one of the major players in the production process of proteins. They are responsible for directing preproteins, a secretory protein with a signal peptide region attached, into a pathway. Its functions are to stop nascent chains mal-folding, recognising and submitting the peptide chain to the secretory machinery and to act as the topological determinant for preproteins in the membrane. In the case of the sec-type signal peptide, they direct pre-proteins from the cytoplasm into the growth medium. Although different signal peptides show little similarity in amino acid sequence, three distinct domains can be distinguished: a positively charged N-terminus (N-region), a central hydrophobic region (H-region) and a polar C-terminal region (C-region). The C-region commonly carries a type-I SPase cleavage site, with the consensus sequence Ala-X-Ala or Val-X-Ala at positions -1 and -3 relative to the cleavage site.
At present, about 60% of all commercially available enzymes are produced by Bacillus bacteria due to their huge capacity for secretion. Heterologous proteins that were successfully secreted by Bacillus bacteria include cutinase, α-amylase, proinsulin etc.
We have shortlisted the following three signal peptides to be transcribed upstream of our gene product:
SacB: MNIKKFAKQATVLTFTTALLAGGATQAFAKET
LipA: MKFVKRRIIALVTILMLSVTSLFALQPSAKAAEH
Epr: MKNMSCKLVVSVTLFFSFLTIGPLAHAQNS
Go back to the >>> Specifications Page >>>
|  "
"