Imperial College/5 September 2008
From 2008.igem.org
| (26 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
|{{#calendar: title=Imperial_College |year=2008 | month=09}} | |{{#calendar: title=Imperial_College |year=2008 | month=09}} | ||
|{{#calendar: title=Imperial_College |year=2008 | month=10}} | |{{#calendar: title=Imperial_College |year=2008 | month=10}} | ||
| - | | rowspan="2" bgcolor=# | + | | rowspan="2" bgcolor=#ffffff width="100%" | |
|} | |} | ||
=5 September 2008= | =5 September 2008= | ||
| - | ==Wetlab== | + | =='''Wetlab'''== |
| - | ==Colony Counting== | + | ===Cloning=== |
| + | |||
| + | * PCR reaction to clone the LacI gene using Pfu set up - standard condtitions and reagents[http://openwetware.org/wiki/IGEM:IMPERIAL/2008/Prototype/Wetlab/PCR]; 56°C for the first 10 cycles, 65°C for the 20 cycles. | ||
| + | *Testing PCR reactions (3) for the Biobrick insert size verification primers (with the pSB1AK3 vector) set up - taq conditions and reagents; at 60°C, 58°C or 56°C for 30 cycles | ||
| + | *GeneArt construct 7, 8, 9 and 11 digests, CAT digest and pSB1A2 digest and the insert (excluding for pSB1A2)purified by gel-purification (pSB1A2 vector minus insert was purified) | ||
| + | *AmyE 5', AmyE 3' and Aad9 PCR reactions digested with ''XbaI'' and ''SpeI'' for cloning into Biobricks and PCR purified | ||
| + | *Terminator Biobrick digested and PCR purified to remove Prefix fragment | ||
| + | |||
| + | ===Colony Counting=== | ||
After re-calibrating our illuminator, it can now count colonies with a fair degree of accuracy. Manual addition and removal of points allows us to fine-tune the results, so we will use this method from now on. | After re-calibrating our illuminator, it can now count colonies with a fair degree of accuracy. Manual addition and removal of points allows us to fine-tune the results, so we will use this method from now on. | ||
| - | ==Trial Run: ''B. subtilis'' Growth Curve== | + | ===Trial Run: ''B. subtilis'' Growth Curve=== |
| - | In our second trial run to obtain growth and calibration curves for ''B. subtilis'', we cultured our "B. subtilis" for two days and | + | In our second trial run to obtain growth and calibration curves for ''B. subtilis'', we cultured our "B. subtilis" for two days and monitored the changes in OD<sub>600</sub> continuously. By plotting OD<sub>600</sub> against time/h, we obtained the following growth curve for "B. subtilis". |
| - | [[Image:Trial_Run_Growth_Curve.png|thumb|600px|center|Growth Curve for ''B. subtilis'']] | + | [[Image:Trial_Run_Growth_Curve.png|thumb|600px|center|Growth Curve for ''B. subtilis'' from trial run]] |
===Calibration Curve=== | ===Calibration Curve=== | ||
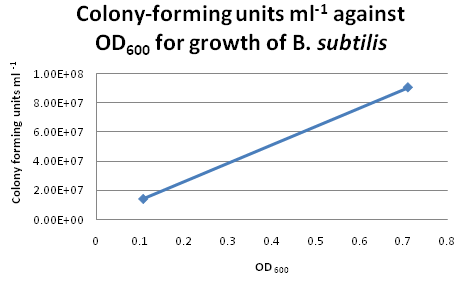
| - | For six of the data points from the above growth curve, we plated 0.1 ml of ''B. subtilis'' on LB agar plates and performed serial | + | For six of the data points from the above growth curve, we plated 0.1 ml of ''B. subtilis'' on LB agar plates and performed serial dilutions in order to calculate the colony-forming units (CFU per ml) for the corresponding OD<sub>600</sub>. However, we only managed to obtain two sets of valid data. The ''B. subtilis'' mixtures we plated for higher values of OD600 were too dilute, hence no bacterial growth was observed on these LB agar plates. |
| - | [[Image:Trial_Run_B_Subtilis_Calibration_Curve.png|thumb|600px|center|Growth Curve for ''B. subtilis'']] | + | [[Image:Trial_Run_B_Subtilis_Calibration_Curve.png|thumb|600px|center|Growth Curve for ''B. subtilis'' from trial run]] |
===Suggestions for Further Improvements=== | ===Suggestions for Further Improvements=== | ||
| - | Pipette tips should be changed... | + | For the official experiment to obtain our calibration curve, we should take the following precautions: |
| + | |||
| + | *Pipette tips should be changed after each transfer of solution during serial dilution. This would avoid contamination and produce more accurate results for the calibration curve. | ||
| + | |||
| + | *Choose dilution factors for plating more carefully. It is not advisable to choose very high dilution factors e.g. those higher than 10E8. | ||
| + | |||
| + | *Check OD<sub>600</sub> readings more frequently during the exponential growth phase. This would ensure a more even distribution of data points for the calibration curve. | ||
| + | |||
| + | =='''Dry Lab'''== | ||
| + | |||
| + | ===Motility=== | ||
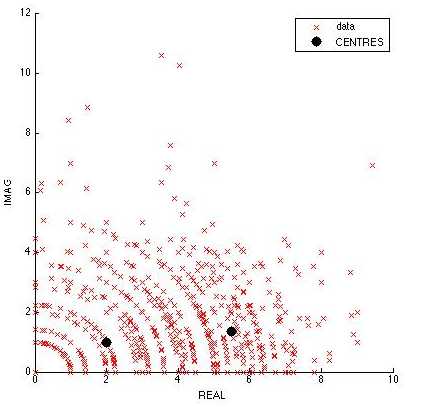
| + | *10 cells were tracked manually from Video 3. The graph of Z=Rexp(i*X) was plotted, R being the displacement and X being the angle between three consecutive coordinate points. It is hypothesised that tumbling will occur with very low displacement and high angle, while running occurs with large displacement and low angle. | ||
| + | *It was found that segmentation could not be done visually. However, using subtractive clustering analysis in the "Find Cluster Toolbox" in MATLAB, we were able to identify 2 centers of clustering, representing tumbling and running. However, more data has to be obtained to visually identify running and tumbling clusters. | ||
| + | [[Image:Cluster.jpg|600px|thumb|center|Complex Plot of Velocity*exp(i*Angle)]] | ||
| + | |||
| + | ===Microscopy=== | ||
| + | 2 microscopy sessions will be arranged for next week, on Wednesday and Friday. | ||
| + | |||
| - | {{Imperial/EndPage}} | + | <br> |
| + | {{Imperial/EndPage|Notebook|Notebook}} | ||
Latest revision as of 20:44, 28 October 2008
5 September 2008WetlabCloning
Colony CountingAfter re-calibrating our illuminator, it can now count colonies with a fair degree of accuracy. Manual addition and removal of points allows us to fine-tune the results, so we will use this method from now on. Trial Run: B. subtilis Growth CurveIn our second trial run to obtain growth and calibration curves for B. subtilis, we cultured our "B. subtilis" for two days and monitored the changes in OD600 continuously. By plotting OD600 against time/h, we obtained the following growth curve for "B. subtilis". Calibration CurveFor six of the data points from the above growth curve, we plated 0.1 ml of B. subtilis on LB agar plates and performed serial dilutions in order to calculate the colony-forming units (CFU per ml) for the corresponding OD600. However, we only managed to obtain two sets of valid data. The B. subtilis mixtures we plated for higher values of OD600 were too dilute, hence no bacterial growth was observed on these LB agar plates. Suggestions for Further ImprovementsFor the official experiment to obtain our calibration curve, we should take the following precautions:
Dry LabMotility
Microscopy2 microscopy sessions will be arranged for next week, on Wednesday and Friday.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"