Team:Chiba/Project/Experiments:Signal Molecule Quencher
From 2008.igem.org
(→Experiments) |
|||
| (4 intermediate revisions not shown) | |||
| Line 34: | Line 34: | ||
'''Detailed Condition''' | '''Detailed Condition''' | ||
| - | Sender; LuxI plasmid transformed into E.coli strains (JW1908).<br> | + | Sender; LuxI plasmid transformed into ''E. coli'' strains (JW1908).<br> |
| - | Receiver; LuxR-gfp plasmid transformed into E.coli strains (JW1908).<br> | + | Receiver; LuxR-gfp plasmid transformed into ''E. coli'' strains (JW1908).<br> |
Culture/ Cndn. | Culture/ Cndn. | ||
| Line 41: | Line 41: | ||
2.Inoculated into flesh media, shaken until cell density hit 2.0 in OD600<br> | 2.Inoculated into flesh media, shaken until cell density hit 2.0 in OD600<br> | ||
3.Washed the cell and re-suspended. Cell density checked.<br> | 3.Washed the cell and re-suspended. Cell density checked.<br> | ||
| - | 4.Mixed Sender and Receiver (Sender/Receiver | + | 4.Mixed Sender and Receiver (Sender/Receiver 1000μL/1000μL).<br> |
5.Incubated at 30°C.<br> | 5.Incubated at 30°C.<br> | ||
6.Time-chased the fluorescence (485nm (excitation) and 527nm (emission)) by gfp.<br> | 6.Time-chased the fluorescence (485nm (excitation) and 527nm (emission)) by gfp.<br> | ||
| Line 64: | Line 64: | ||
===Method=== | ===Method=== | ||
| - | #Transformed Sender into E.coli strains(JW1908) and Receivers into E.coli strain(JW1908). | + | #Transformed Sender into ''E. coli'' strains (JW1908) and Receivers into ''E. coli'' strain (JW1908). |
#Inoculated them independently in liquid media. Incubated at 37°C 12h. | #Inoculated them independently in liquid media. Incubated at 37°C 12h. | ||
#Inoculated again at 37°C upto about OD600=2.0 | #Inoculated again at 37°C upto about OD600=2.0 | ||
Latest revision as of 09:35, 30 October 2008
| Home | The Team | The Project | Parts Submitted to the Registry | Reference | Notebook | Acknowledgements |
|---|
Partial quenching of signals (jamming)
Design
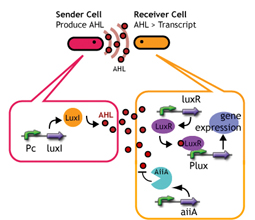
AiiA added to Lux reporter
We introduced AiiA [http://www.genome.jp/dbget-bin/www_bget?enzyme+3.1.1.81 (enzyme+3.1.1.81)] (the autoinducer inactivation enzyme A from Bacillus) to LuxR receiver. AiiA is an enzyme that degrades AHL (1) (Fig. 2). The idea was that the effective concentration of AHL in receiver cell get significantly decreased due to the constantly expressing AiiA, thereby enlarging the delay time. Fluorescence should be observed only when [AHL] exceeds the degrading capacity of aiiA.
Experiments
Co-transformation
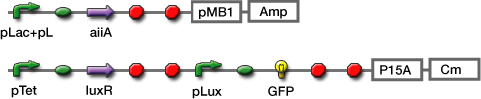
We constructed the circuit below. AiiA is placed on the high-copy plasmid under the control of Lac promoter. It was co-transformed with the LuxR/ Plux reporter on the p15A plasmid.
Communication
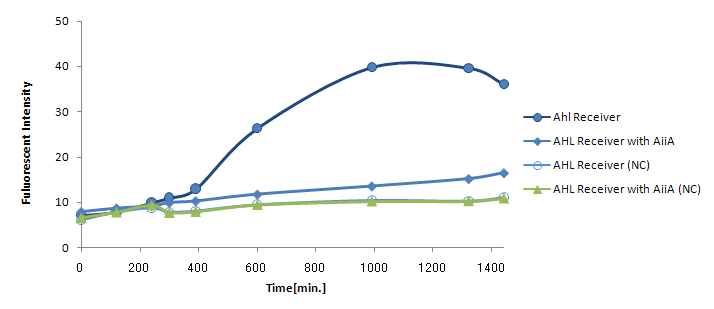
The resultant "AiiA/LuxR reporter" was co-cultured with LuxI sender and the fluorescence was monitored over time. As a control, we conducted the same experiment with LuxR reporter without AiiA plasmid.
Detailed Condition
Sender; LuxI plasmid transformed into E. coli strains (JW1908).
Receiver; LuxR-gfp plasmid transformed into E. coli strains (JW1908).
Culture/ Cndn.
1.Both Sender and Receiver (+/- AiiA) were inoculated into small (2mL) culture and was shaken separately for 12h (at 37°C)
2.Inoculated into flesh media, shaken until cell density hit 2.0 in OD600
3.Washed the cell and re-suspended. Cell density checked.
4.Mixed Sender and Receiver (Sender/Receiver 1000μL/1000μL).
5.Incubated at 30°C.
6.Time-chased the fluorescence (485nm (excitation) and 527nm (emission)) by gfp.
|
|
| [http://partsregistry.org/Part:BBa_S03623 BBa_S03623 (AHL sender)] |
|
|
Method
- Transformed Sender into E. coli strains (JW1908) and Receivers into E. coli strain (JW1908).
- Inoculated them independently in liquid media. Incubated at 37°C 12h.
- Inoculated again at 37°C upto about OD600=2.0
- Washed them.
- Mixed them (Sender:Receiver=1000μl:1000μl).
- Incubated at 30°C.
- Measured intensity of green fluorescence at regular time intervals.
Result & Discussion
The co-expression of AiiA resulted in the drastic decrease in the fluorescence all through the experiment. It hasn't reached the endpoint even 24h after mixing. AiiA looks super-active and consume the most AHL molecule out; Obviously, the AHL activity is way too much.
On the other hand, we observed gradual increase in fluorescence over time. At least, the fluorescence from the co-culture was always above the negative control (without Lux-Sender). This indicate AiiA is not eating up the all signaling molecule. If we properly down-tune the AiiA activity (either by putting this gene into low-copy plasmid or by giving the low efficiency rbs), we should be able to see the time-delay.
References
- [http://www.jbc.org/cgi/content/full/279/14/13645 Wang et al.:Specificity and Enzyme Kinetics of the Quorum-quenching N-Acyl Homoserine Lactone Lactonase (AHL-lactonase).J. Biol. Chem.279(14),13645-13651,2004.]
| Home | The Team | The Project | Parts Submitted to the Registry | Notebook |
|---|
 "
"