Team:EPF-Lausanne/Project
From 2008.igem.org
(→General scheme) |
(→Results) |
||
| (140 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | < | + | {{EPFL/Header}} |
| + | <br> | ||
| + | <div style="font-size:20pt;"><center>Genetic Network Generating Spatial Patterns Through Cell-Cell Communication</center></div> | ||
| + | <br> | ||
| + | <div style="font-size:14pt;"><center> </center></div> | ||
| + | <div style="font-size:20pt;"><center>and Controlled Information Processing</center></div><br> | ||
| + | == Abstract == | ||
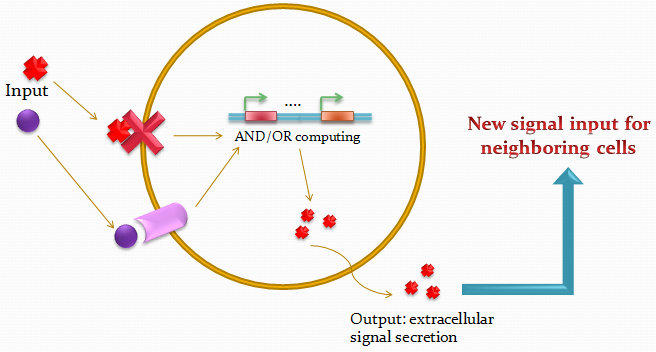
| - | + | Biological systems are unique in their ability to combine information and energy to generate complex entities. Genetically encoded networks drive many of these patterning processes. Furthermore, developmental studies have highlighted the importance of gradient formation and cell-cell communication for the generation of cellular patterns in the early stages of life. It has been shown that simple networks can form both static and dynamic patterns. Nonetheless, a system whose pattern formation is dependent on combinations of multiple signals has yet to be demonstrated. Here we address this question by designing a network, involving two different quorum-sensing based signaling mechanisms. Upon introduction in ''E.coli'', the system can sense the relative amounts of two input molecules. Using a pre-defined set of rules, which was selected on its ability to generate spatial patterns, the cell can then express its final state by emitting red or green fluorescence and transmit its state to its neighbors. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | [[Image:general concept2.png|center]] | |
| + | == Towards an in-vivo self organizing system...== | ||
| + | In recent years, quorum sensing has been of wide-spread interest in synthetic biology. The ability for populations of cells to communicate in such a way that they either relay information or create specific patterns in culture is one which has been used in several studies. Some have relied on two different cell populations (BALAGADDE ''et al'', 2008; BASU ''et al'', 2005) to engineer novel in vitro behavior. | ||
| - | + | The idea of the project is to build a system which uses two different quorum sensing systems of molecules that enable the cell to integrate three different levels of signal, and emit a different response according to a predetermined rule-set, producing a given fluorescent protein and a quorum sensing molecule. | |
| - | + | ||
| - | + | Our project uses a single population of cells, which all contain the same genetic information. Thus, the different behavior of the sub-populations will only be dictated by neighboring sub-populations. | |
| - | + | ||
| - | + | We also take advantage of the high-level engineering competences found in our Institute (EPFL) by using microfluidic devices in order to culture the cells in an ordered and controllable manner. Furthermore, this will also permit to have control on the information flux dynamics between the cells. | |
| - | '' | + | |
| - | + | In other words, we want to implement an '''in-vivo self organizing system'''. The cell population is '''self-organized''' in the sense that, unlike other experimental setups, no artificial gradient nor sender cells are required to generate a pattern. In these other situations, the cells are placed in different | |
| + | conditions at the initial stages of the experiment. | ||
| + | |||
| + | In our idealized case, if a perturbation is applied to a subpart of the population, the same pattern can be generated regardless of what subpart of the cell population was actually subjected to this perturbation. | ||
| + | Now the microfluidics-based control on the information flux across the population is not in essence contradictory to the "self organized" nature desired for our system, considering that the use of this control will only depend on the state of the cells. In other words, control on the signaling is equivalent to choosing a cell-cell communication protocol. | ||
| + | |||
| + | |||
| + | Our devices are custom-made for the project, fabricated using PDMS and multilayer soft lithography (MSL). | ||
| + | We designed an array of miniature chambers to culture cells. The chambers are linked to one another with a network of ducts enabling us to culture sub-populations of our cells in contact with each other. Depending on the chamber connectivity and the genetic rule-set used, we expect complex patterns of GFP/RFP expression to form (see patterns below). | ||
| + | |||
| + | The aim is to initiate a signal on one side of the channel and observe the propagation along the plate. | ||
| + | |||
| + | {| border ="0" style="background:transparent;" | ||
|- | |- | ||
| | | | ||
| - | | | + | [[Image:initiation.png|300px|left]] |
| + | || | ||
| + | [[Image:establishment.png|365px|left]] | ||
|} | |} | ||
| - | |||
| - | + | Our microfluidic devices are also used in a side-project in order to characterize the binding site specificity and affinity of quorum sensing-related transcription factors (the MITOMI devices). | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ( | + | |
| + | == Biomedical application == | ||
| + | Our system could be further developed to function as a diagnostics device detecting disease-related biomarkers in bodily fluids. Each first column chip well would contain specialized bacterial cells engineered to detect specific biomarkers, allowing the simultaneous sampling of hundreds to even thousands of patient samples. Depending on which samples contain the biomarker of interest, a specific pattern would be generated. Using our algorithm, we would have a priori defined all possible patterns generated by all potential combinations of positive wells. Simple scanning and analysis of the pattern by a computer would then automatically identify which wells are positive, providing a cost-effective and high-throughput mechanism to analyze patient samples. | ||
| - | == | + | ==Initiation of the project== |
| + | Our goal was to produce a system as simple as possible giving patterns as complex as possible. | ||
| + | We therefore decided to use only two quorum sensing systems - and two fluorescent proteins - while testing various network geometries and rule sets. | ||
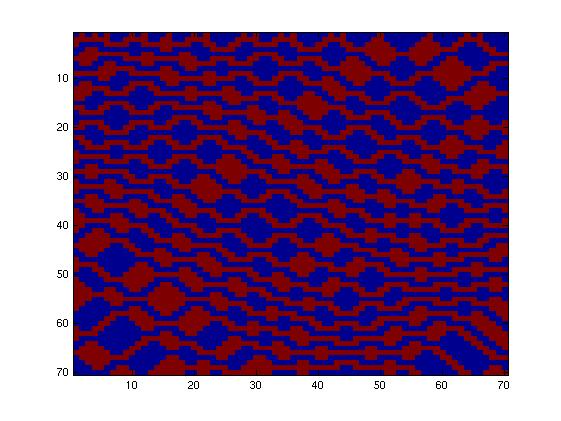
| + | We tried two different fluidic networks, determining the patterns formed by all possible rule-sets. We mean by "rule-set" the law determining the output response of the system to a given input. | ||
| - | |||
| - | The | + | The first fluidic network, with simple connections, gave two simple patterns, like the "barcode" one. |
| - | + | ||
| - | + | [[Image:connection2.png|250px]] | |
| + | [[Image:5050.jpg|250px]] | ||
| - | |||
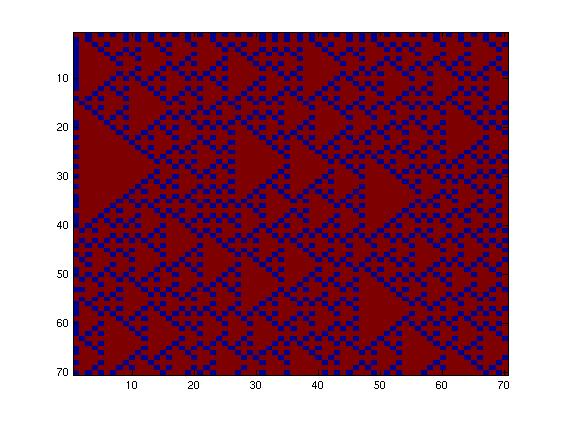
| - | + | The second network, with more complex connectivity, gave more interesting patterns like the "triangles" one. | |
| + | |||
| + | [[Image:connection1b.png|250px]] | ||
| + | [[Image:Fullpatches_1010.jpg|250px]] | ||
| + | [[Image:Triangles.jpg|250px]] | ||
| + | |||
| + | |||
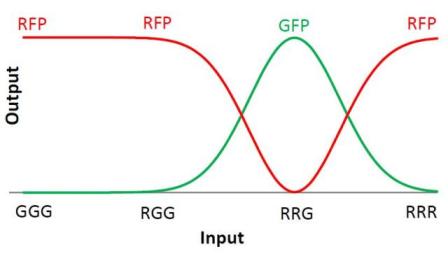
| + | We choose to keep the "triangles" pattern, and to try to implement its rule set, given in the following table. You can see the desired expression level of each fluorescent protein on the graph below. | ||
| + | |||
| + | |||
| + | {| border="1" cellpadding="5" align="center" | ||
| + | |- | ||
| + | ! width="50"|Input || width="50"|Output | ||
| + | |- | ||
| + | | GGG || R | ||
| + | |- | ||
| + | | RGG || R | ||
| + | |- | ||
| + | | RRG || G | ||
| + | |- | ||
| + | | RRR || R | ||
| + | |} | ||
| + | |||
| + | |||
| + | The rules in this table are what we tried to implement in our system. When the cell receives a given input, it will respond by producing the correct output. | ||
| + | |||
| + | |||
| + | [[Image:Rule6.JPG|center]] | ||
== Project Details== | == Project Details== | ||
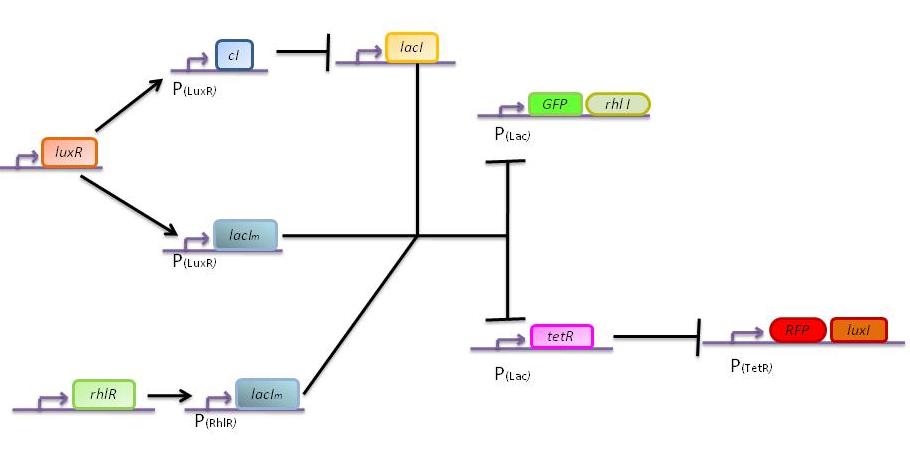
| - | + | '''General scheme :''' | |
| - | [[Image: | + | The genetic circuit which will be implemented in the cells is the following : |
| + | |||
| + | [[Image:finnewscheme.png|center]] | ||
| + | |||
| + | '''Pattern:''' | ||
This genetic circuit must give this following pattern : | This genetic circuit must give this following pattern : | ||
| - | [[Image:Triangles.jpg]] | + | [[Image:Triangles.jpg|center]] |
| + | '''Plasmids:''' | ||
| - | + | Our system introduces a new plasmid in a pre-existing system ("A synthetic multicellular system for | |
| - | Our system | + | programmed pattern formation", from Ron Weiss et al., Nature, vol.434, April 2005) which detects different levels of the luxI quorum sensing molecule and gives a specific response according to the detected or observed concentration . As shown on the previous genetic circuit, we will clone the gene coding for the RhlI molecule at the 3' end of the ''GFP'' gene, with C4-HSL being our second quorum sensing molecule used. |
| - | programmed pattern formation", from Ron Weiss et al., Nature, vol.434, April 2005) which | + | In addition to the two Ron Weiss system plasmids, we have to build an additional plasmid for the second part of the proposed genetic circuit. We will add an extra copy of the ''lacIm'' gene from the Ron Weiss system so that we can test it independently from the latter system. |
| - | In addition to the two Ron Weiss system plasmids, we have to build an | + | |
| - | Here is the scheme of the plasmid with the Registry of Standard Parts references for each Biobrick we | + | Here is the scheme of the plasmid with the Registry of Standard Parts references for each Biobrick that is used. |
| - | [[Image: | + | [[Image:PiGEM3.png|center]] |
| + | |||
| + | '''Response:''' | ||
| + | |||
| + | The response of our network depends on the incoming signal. Four cases can be found : | ||
| + | |||
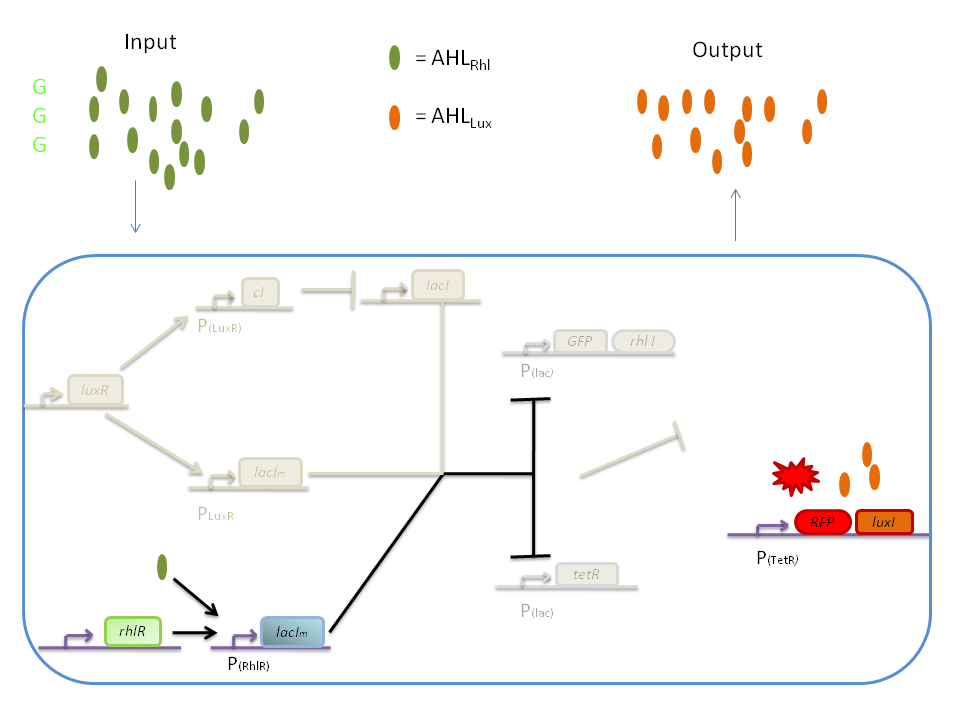
| + | '''1) GGG''' | ||
| + | |||
| + | If there is green but no red, the AHL<SUB>Rhl</SUB> will bind to the RhlR to allow transcription of ''lacI<SUB>m</SUB>'' . This LacI<SUB>m</SUB> will inhibit transcription of both the ''GFP'' and the ''tetR'' genes. This latter inhibition then allows the transcription of the ''RFP'' and ''luxI'' genes. | ||
| + | |||
| + | [[Image:GGG.png|800px|center]] | ||
| + | |||
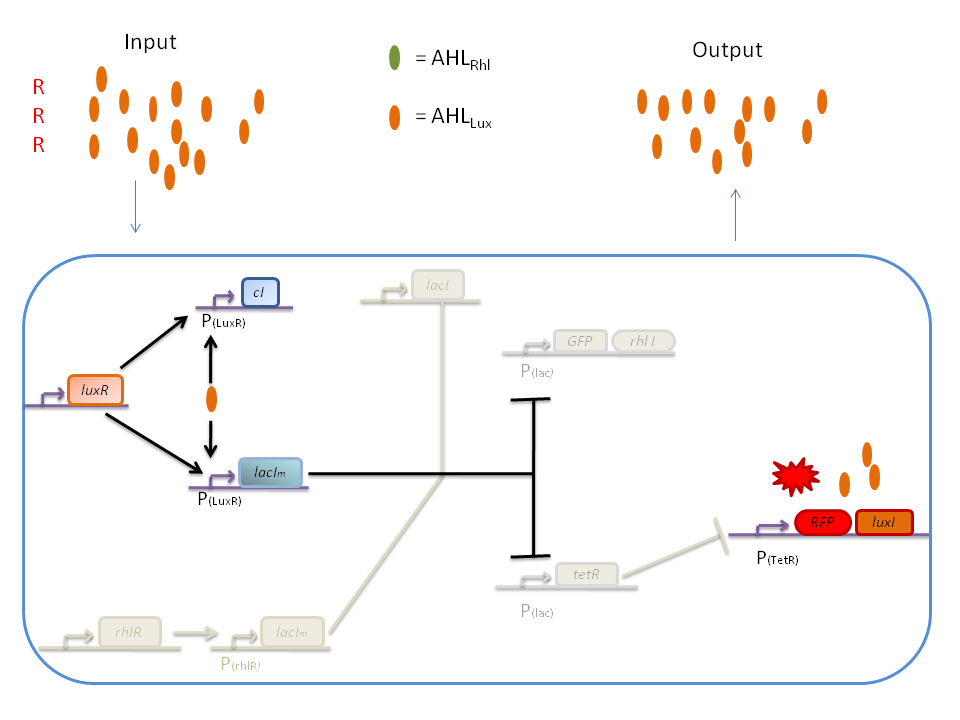
| + | '''2) RRR''' | ||
| + | |||
| + | If there is a high level of red and no green, this means that there is a lot of AHL<SUB>Lux</SUB> which will bind to the LuxR and allow the transcription of ''lacIm'' and ''cI''. cI will then inhibit ''lacI'', but LacI<SUB>m</SUB> will inhibit on the one hand the ''GFP'' (leading to no GFP production) and on the other hand ''tetR''. Thus, as no TetR is produced, RFP will be produced. | ||
| + | |||
| + | [[Image:RRR.png|800px|center]] | ||
| + | |||
| + | |||
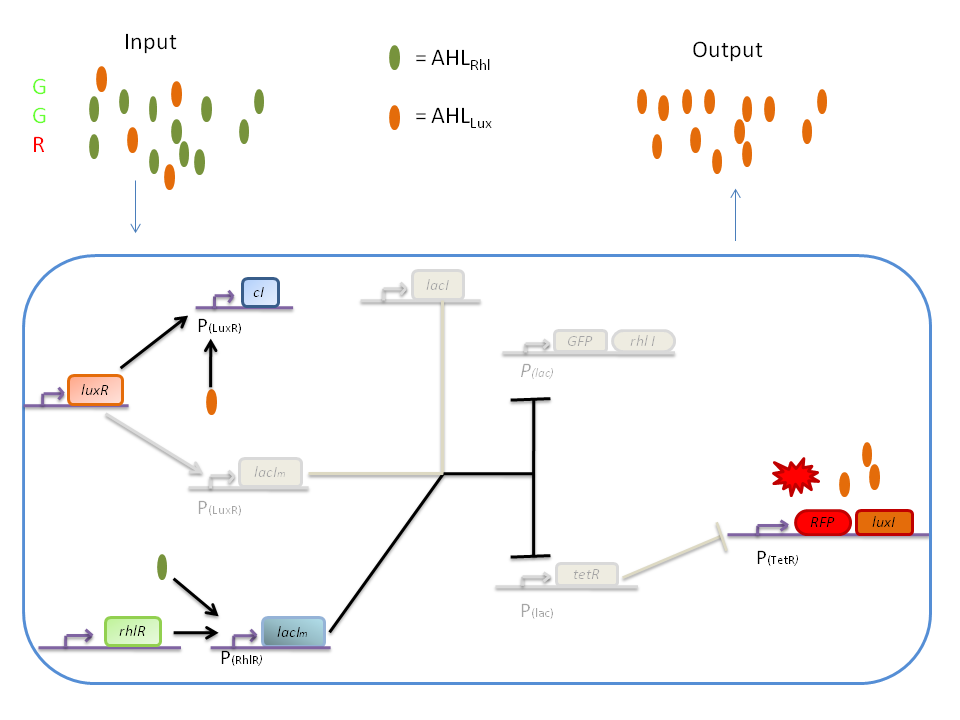
| + | '''3) GGR''' | ||
| + | |||
| + | When both AHL<SUB>Rhl</SUB> and AHL<SUB>Lux</SUB> are present, we need to consider the influence of each molecule. | ||
| + | The input of a green state means that AHL<SUB>Rhl</SUB> will also be present, which will bind to the RhlR allowing the transcription of ''lacI<SUB>m</SUB>''. This one will inhibit ''GFP'' and also the ''tetR'', leading to the production of RFP. | ||
| + | The low input of the red state means that there is also a low level of AHL<SUB>Lux</SUB>. This AHL<SUB>Lux</SUB> will bind to LuxR to produce cI and inhibit ''lacI''. But, LacIm initiated by the ''rhlR'' promoter will remain in the cell and repress ''GFP'' and ''tetR'', therefore leading to the production of RFP and LuxI. | ||
| + | |||
| + | [[Image:GGR.png|800px|center]] | ||
| + | |||
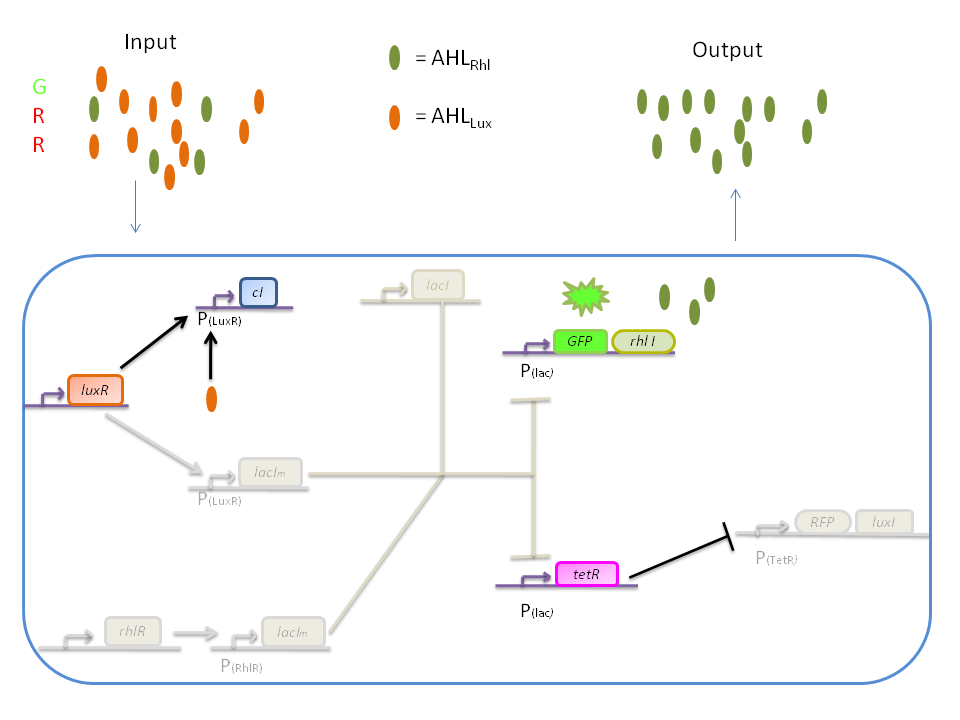
| + | '''4) GRR''' | ||
| + | |||
| + | When both AHL<SUB>Rhl</SUB> and AHL<SUB>Lux</SUB> are present, we need to consider once more the influence of each molecule. | ||
| + | Here the green input is weak and AHL<SUB>Rhl</SUB> concentration is low. Therefore, the AHLRhl concentration will not be enough to express LacIm. | ||
| + | The medium input of red means that AHL<SUB>Lux</SUB> is present in a concentration which is within the range of the band-pass. In that case, AHL<SUB>Lux</SUB> will bind to LuxR to produce cI but will not activate ''lacI<SUB>m</SUB>''. The cI will inhibit ''lacI'', leading to the production of GFP and TetR. The latter will finally repress the ''RFP'' leading to a final green state with production of RhlI. | ||
| + | |||
| + | [[Image:GRR.png|800px|center]] | ||
== Experiments == | == Experiments == | ||
| + | |||
| + | In order to complete our project, we followed mainly three courses of action. The first focuses on the network design and the mathematical modeling; it aims to draw the principles of information processing by individual cells and the cell population, with ODEs used to model the reaction of the system. Details on this part are available in the corresponding page of the Wiki : [https://2008.igem.org/Team:EPF-Lausanne/Modeling Modeling]. This part was done with the help of professor Felix Naef. | ||
| + | |||
| + | The second part of the project was of course the concrete generation of the required plasmids in the laboratory, which was executed in the facilities provided by the Laboratory of Systems Biology and Genetics (http://deplanckelab.epfl.ch/) of Prof. Bart Deplancke. Using Biobricks and material obtained from the Ron Weiss group, we followed a cloning scheme to obtain our genetic circuit in ''E. coli''. | ||
| + | |||
| + | Most of the information on the cloning can be found on the [https://2008.igem.org/Team:EPF-Lausanne/Parts Parts] section of this Wiki, with respect to the parts submitted, on this Project page higher up (description of the plasmid), and in the [https://2008.igem.org/Team:EPF-Lausanne/Notebook Notebook] section for the protocols used in culture, transformation, digestion and ligation. | ||
| + | |||
| + | The third part of the project consisted in the building and using of the microfluidic chips for the two sides of the project in the Laboratory of Biological Network Characterization (http://lbnc.epfl.ch/) of Prof. Sebastian Maerkl. First, the MITOMI approach was implemented in the laboratory to characterize the DNA binding specificity and affinity of quorum sensing-related transcription factors . Then, the chips for cell culture and test of the genetic circuit were designed. Information on this part of the project can be found on the corresponding page of the Wiki : [https://2008.igem.org/Team:EPF-Lausanne/_Microfluidics Microfluidics]. Protocols for the fabrication of the devices and the preparation of DNA and transcription factors can also be found on the [https://2008.igem.org/Team:EPF-Lausanne/Notebook Notebook] page of this Wiki. | ||
== Results == | == Results == | ||
| + | |||
| + | The part BBa_K092600, which is the construct shown below, consists of the gene for the TetR signaling protein and the RFP encoding sequence under TetR control but without the PLac promoter which regulates the expression of TetR. This part was characterized. | ||
| + | |||
| + | |||
| + | [[Image:Testpart.jpg|center]] | ||
| + | |||
| + | Theoretically, this construct should not be able to express RFP due to the fact that the promoter for the induction molecule (TetR) is not present and thus no TetR should be expressed. | ||
| + | |||
| + | However, preliminary experiments indicated that TetR expression is leaky and we therefore exploited this feature to characterize the respective part(see protocol in parts registry BBa_K092600). We found that RPF was indeed produced thus indicating a basal ‘leaky’ expression, and this allowed us to predict that the addition of the PLac promotor should highly enhance RFP production. | ||
| + | |||
| + | Specifically, we obtained a transfer function that is shown below. | ||
| + | |||
| + | [[Image:Transfer_function.jpg|center]] | ||
| + | |||
| + | Flow cytometry was carried out to verify the presence of RFP expressing cells. After the plate reading assay, the same samples were taken for flow cytometry. Below, the flow cytometry results for samples at two high concentrations is shown. In the histograms below, we can clearly see the shift in the different cell types, namely between those that produce and do not produce RFP. In the x-axis is the log of fluorescence intensity, and in the y-axis the cell count. | ||
| + | |||
| + | [[Image:Cytometer.jpg|center]] | ||
| + | These results demonstrate that the construct that was made does indeed function and that upon completion can yield high levels of RFP. At the same time though, such production of RFP may interfere with the precise control that is required in our system. | ||
| + | |||
| + | We were not able to test the entire circuit because the final ligations were not completed in time. But the obtained results indicate, as noted before, that the genetic circuit that was conceived can be functional upon completion of the final construct. | ||
| + | |||
| + | '''Summary''' | ||
| + | |||
| + | At the time of the Wiki deadline, we were one step away from finishing both plasmids necessary to complete our synthetic network. All subparts have been sequenced and some have been characterized (TetR, RFP). Work is also still being continued at the EPFL to finish the cloning and realize a first full scale on-chip experiment. | ||
| + | The microfluidic chip can be successfully loaded with a high cell density. Even though some cells die in the chip, most of them survive and can express a fluorescent marker (GFP or RFP). The main issue resides in the small amount of available nutrient on the chip. On the other hand, the valves work nicely and allow an accurate control of quorum-sensing molecules concentration throughout the chip. | ||
| + | |||
| + | On the modeling side, simulations have been run and showed that the network can respond to green and red levels according to our set of rules. The model showed that our system is very sensitive to many parameters as well as to the level of the molecular components available at the time of the external AHL input. Considering the very complex and noisy "milieu" of the cell, we might think that our network is not robust enough to produce the expected functionality. It was quite challenging to design a self-organizing system made of one unique cell type. As a result, the designed network is highly connected and very complex with many feedback and feedforward loops. | ||
| + | |||
| + | Undergoing this iGEM project, we wanted to tackle one fundamental question about the emergence of life: "How can a complex organism arise from one single cell?" It seems clear that such complexity cannot be implemented in a synthetic system. Nevertheless, we believe that synthetic biology might bring interesting insights into the functionality of self-organizing population of cells. | ||
| + | {{EPFL/Header}} | ||
Latest revision as of 23:12, 29 October 2008
| Home | The Team | The Project | Parts | 2-step PCR | Microfluidics | Modeling | Notebook |
|---|
Contents |
Abstract
Biological systems are unique in their ability to combine information and energy to generate complex entities. Genetically encoded networks drive many of these patterning processes. Furthermore, developmental studies have highlighted the importance of gradient formation and cell-cell communication for the generation of cellular patterns in the early stages of life. It has been shown that simple networks can form both static and dynamic patterns. Nonetheless, a system whose pattern formation is dependent on combinations of multiple signals has yet to be demonstrated. Here we address this question by designing a network, involving two different quorum-sensing based signaling mechanisms. Upon introduction in E.coli, the system can sense the relative amounts of two input molecules. Using a pre-defined set of rules, which was selected on its ability to generate spatial patterns, the cell can then express its final state by emitting red or green fluorescence and transmit its state to its neighbors.
Towards an in-vivo self organizing system...
In recent years, quorum sensing has been of wide-spread interest in synthetic biology. The ability for populations of cells to communicate in such a way that they either relay information or create specific patterns in culture is one which has been used in several studies. Some have relied on two different cell populations (BALAGADDE et al, 2008; BASU et al, 2005) to engineer novel in vitro behavior.
The idea of the project is to build a system which uses two different quorum sensing systems of molecules that enable the cell to integrate three different levels of signal, and emit a different response according to a predetermined rule-set, producing a given fluorescent protein and a quorum sensing molecule.
Our project uses a single population of cells, which all contain the same genetic information. Thus, the different behavior of the sub-populations will only be dictated by neighboring sub-populations.
We also take advantage of the high-level engineering competences found in our Institute (EPFL) by using microfluidic devices in order to culture the cells in an ordered and controllable manner. Furthermore, this will also permit to have control on the information flux dynamics between the cells.
In other words, we want to implement an in-vivo self organizing system. The cell population is self-organized in the sense that, unlike other experimental setups, no artificial gradient nor sender cells are required to generate a pattern. In these other situations, the cells are placed in different conditions at the initial stages of the experiment.
In our idealized case, if a perturbation is applied to a subpart of the population, the same pattern can be generated regardless of what subpart of the cell population was actually subjected to this perturbation. Now the microfluidics-based control on the information flux across the population is not in essence contradictory to the "self organized" nature desired for our system, considering that the use of this control will only depend on the state of the cells. In other words, control on the signaling is equivalent to choosing a cell-cell communication protocol.
Our devices are custom-made for the project, fabricated using PDMS and multilayer soft lithography (MSL).
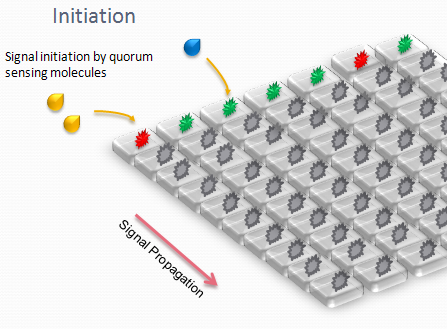
We designed an array of miniature chambers to culture cells. The chambers are linked to one another with a network of ducts enabling us to culture sub-populations of our cells in contact with each other. Depending on the chamber connectivity and the genetic rule-set used, we expect complex patterns of GFP/RFP expression to form (see patterns below).
The aim is to initiate a signal on one side of the channel and observe the propagation along the plate.
Our microfluidic devices are also used in a side-project in order to characterize the binding site specificity and affinity of quorum sensing-related transcription factors (the MITOMI devices).
Biomedical application
Our system could be further developed to function as a diagnostics device detecting disease-related biomarkers in bodily fluids. Each first column chip well would contain specialized bacterial cells engineered to detect specific biomarkers, allowing the simultaneous sampling of hundreds to even thousands of patient samples. Depending on which samples contain the biomarker of interest, a specific pattern would be generated. Using our algorithm, we would have a priori defined all possible patterns generated by all potential combinations of positive wells. Simple scanning and analysis of the pattern by a computer would then automatically identify which wells are positive, providing a cost-effective and high-throughput mechanism to analyze patient samples.
Initiation of the project
Our goal was to produce a system as simple as possible giving patterns as complex as possible. We therefore decided to use only two quorum sensing systems - and two fluorescent proteins - while testing various network geometries and rule sets. We tried two different fluidic networks, determining the patterns formed by all possible rule-sets. We mean by "rule-set" the law determining the output response of the system to a given input.
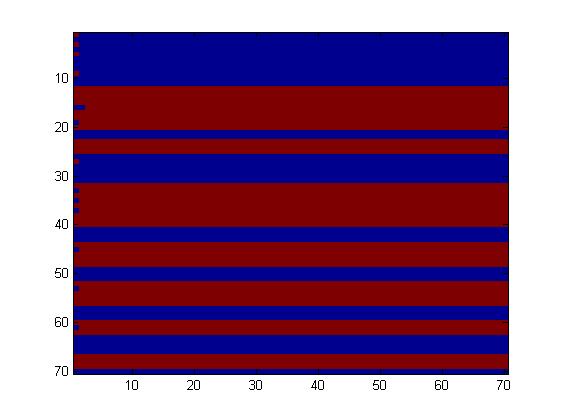
The first fluidic network, with simple connections, gave two simple patterns, like the "barcode" one.
The second network, with more complex connectivity, gave more interesting patterns like the "triangles" one.
We choose to keep the "triangles" pattern, and to try to implement its rule set, given in the following table. You can see the desired expression level of each fluorescent protein on the graph below.
| Input | Output |
|---|---|
| GGG | R |
| RGG | R |
| RRG | G |
| RRR | R |
The rules in this table are what we tried to implement in our system. When the cell receives a given input, it will respond by producing the correct output.
Project Details
General scheme :
The genetic circuit which will be implemented in the cells is the following :
Pattern:
This genetic circuit must give this following pattern :
Plasmids:
Our system introduces a new plasmid in a pre-existing system ("A synthetic multicellular system for programmed pattern formation", from Ron Weiss et al., Nature, vol.434, April 2005) which detects different levels of the luxI quorum sensing molecule and gives a specific response according to the detected or observed concentration . As shown on the previous genetic circuit, we will clone the gene coding for the RhlI molecule at the 3' end of the GFP gene, with C4-HSL being our second quorum sensing molecule used. In addition to the two Ron Weiss system plasmids, we have to build an additional plasmid for the second part of the proposed genetic circuit. We will add an extra copy of the lacIm gene from the Ron Weiss system so that we can test it independently from the latter system.
Here is the scheme of the plasmid with the Registry of Standard Parts references for each Biobrick that is used.
Response:
The response of our network depends on the incoming signal. Four cases can be found :
1) GGG
If there is green but no red, the AHLRhl will bind to the RhlR to allow transcription of lacIm . This LacIm will inhibit transcription of both the GFP and the tetR genes. This latter inhibition then allows the transcription of the RFP and luxI genes.
2) RRR
If there is a high level of red and no green, this means that there is a lot of AHLLux which will bind to the LuxR and allow the transcription of lacIm and cI. cI will then inhibit lacI, but LacIm will inhibit on the one hand the GFP (leading to no GFP production) and on the other hand tetR. Thus, as no TetR is produced, RFP will be produced.
3) GGR
When both AHLRhl and AHLLux are present, we need to consider the influence of each molecule. The input of a green state means that AHLRhl will also be present, which will bind to the RhlR allowing the transcription of lacIm. This one will inhibit GFP and also the tetR, leading to the production of RFP. The low input of the red state means that there is also a low level of AHLLux. This AHLLux will bind to LuxR to produce cI and inhibit lacI. But, LacIm initiated by the rhlR promoter will remain in the cell and repress GFP and tetR, therefore leading to the production of RFP and LuxI.
4) GRR
When both AHLRhl and AHLLux are present, we need to consider once more the influence of each molecule. Here the green input is weak and AHLRhl concentration is low. Therefore, the AHLRhl concentration will not be enough to express LacIm. The medium input of red means that AHLLux is present in a concentration which is within the range of the band-pass. In that case, AHLLux will bind to LuxR to produce cI but will not activate lacIm. The cI will inhibit lacI, leading to the production of GFP and TetR. The latter will finally repress the RFP leading to a final green state with production of RhlI.
Experiments
In order to complete our project, we followed mainly three courses of action. The first focuses on the network design and the mathematical modeling; it aims to draw the principles of information processing by individual cells and the cell population, with ODEs used to model the reaction of the system. Details on this part are available in the corresponding page of the Wiki : Modeling. This part was done with the help of professor Felix Naef.
The second part of the project was of course the concrete generation of the required plasmids in the laboratory, which was executed in the facilities provided by the Laboratory of Systems Biology and Genetics (http://deplanckelab.epfl.ch/) of Prof. Bart Deplancke. Using Biobricks and material obtained from the Ron Weiss group, we followed a cloning scheme to obtain our genetic circuit in E. coli.
Most of the information on the cloning can be found on the Parts section of this Wiki, with respect to the parts submitted, on this Project page higher up (description of the plasmid), and in the Notebook section for the protocols used in culture, transformation, digestion and ligation.
The third part of the project consisted in the building and using of the microfluidic chips for the two sides of the project in the Laboratory of Biological Network Characterization (http://lbnc.epfl.ch/) of Prof. Sebastian Maerkl. First, the MITOMI approach was implemented in the laboratory to characterize the DNA binding specificity and affinity of quorum sensing-related transcription factors . Then, the chips for cell culture and test of the genetic circuit were designed. Information on this part of the project can be found on the corresponding page of the Wiki : Microfluidics. Protocols for the fabrication of the devices and the preparation of DNA and transcription factors can also be found on the Notebook page of this Wiki.
Results
The part BBa_K092600, which is the construct shown below, consists of the gene for the TetR signaling protein and the RFP encoding sequence under TetR control but without the PLac promoter which regulates the expression of TetR. This part was characterized.
Theoretically, this construct should not be able to express RFP due to the fact that the promoter for the induction molecule (TetR) is not present and thus no TetR should be expressed.
However, preliminary experiments indicated that TetR expression is leaky and we therefore exploited this feature to characterize the respective part(see protocol in parts registry BBa_K092600). We found that RPF was indeed produced thus indicating a basal ‘leaky’ expression, and this allowed us to predict that the addition of the PLac promotor should highly enhance RFP production.
Specifically, we obtained a transfer function that is shown below.
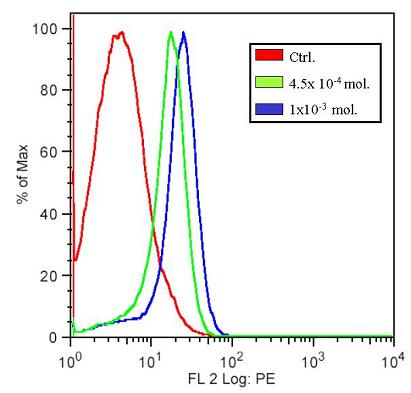
Flow cytometry was carried out to verify the presence of RFP expressing cells. After the plate reading assay, the same samples were taken for flow cytometry. Below, the flow cytometry results for samples at two high concentrations is shown. In the histograms below, we can clearly see the shift in the different cell types, namely between those that produce and do not produce RFP. In the x-axis is the log of fluorescence intensity, and in the y-axis the cell count.
These results demonstrate that the construct that was made does indeed function and that upon completion can yield high levels of RFP. At the same time though, such production of RFP may interfere with the precise control that is required in our system.
We were not able to test the entire circuit because the final ligations were not completed in time. But the obtained results indicate, as noted before, that the genetic circuit that was conceived can be functional upon completion of the final construct.
Summary
At the time of the Wiki deadline, we were one step away from finishing both plasmids necessary to complete our synthetic network. All subparts have been sequenced and some have been characterized (TetR, RFP). Work is also still being continued at the EPFL to finish the cloning and realize a first full scale on-chip experiment. The microfluidic chip can be successfully loaded with a high cell density. Even though some cells die in the chip, most of them survive and can express a fluorescent marker (GFP or RFP). The main issue resides in the small amount of available nutrient on the chip. On the other hand, the valves work nicely and allow an accurate control of quorum-sensing molecules concentration throughout the chip.
On the modeling side, simulations have been run and showed that the network can respond to green and red levels according to our set of rules. The model showed that our system is very sensitive to many parameters as well as to the level of the molecular components available at the time of the external AHL input. Considering the very complex and noisy "milieu" of the cell, we might think that our network is not robust enough to produce the expected functionality. It was quite challenging to design a self-organizing system made of one unique cell type. As a result, the designed network is highly connected and very complex with many feedback and feedforward loops.
Undergoing this iGEM project, we wanted to tackle one fundamental question about the emergence of life: "How can a complex organism arise from one single cell?" It seems clear that such complexity cannot be implemented in a synthetic system. Nevertheless, we believe that synthetic biology might bring interesting insights into the functionality of self-organizing population of cells.
| Home | The Team | The Project | Parts | 2-step PCR | Microfluidics | Modeling | Notebook |
|---|
 "
"