Team:BCCS-Bristol/Calendar-Notebook/21 July 2008
From 2008.igem.org
(→OD measurement) |
JGSchenkel (Talk | contribs) |
||
| (15 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | <html><link rel="stylesheet" href="http://homepage.mac.com/tgorochowski/iGEM/bccs-igem.css" type="text/css"></html> | |
| - | + | __NOTOC__ | |
| + | <div class="bccsNavBar"> | ||
{| align="center" | {| align="center" | ||
!align="center"|[[Team:BCCS-Bristol|Home]] | !align="center"|[[Team:BCCS-Bristol|Home]] | ||
| Line 11: | Line 12: | ||
!align="center"|[[Team:BCCS-Bristol/Misc|Miscellaneous]] | !align="center"|[[Team:BCCS-Bristol/Misc|Miscellaneous]] | ||
|} | |} | ||
| + | <br> | ||
| + | </div> | ||
| - | |||
| - | |||
| - | + | == '''Growth curve measurement''' == | |
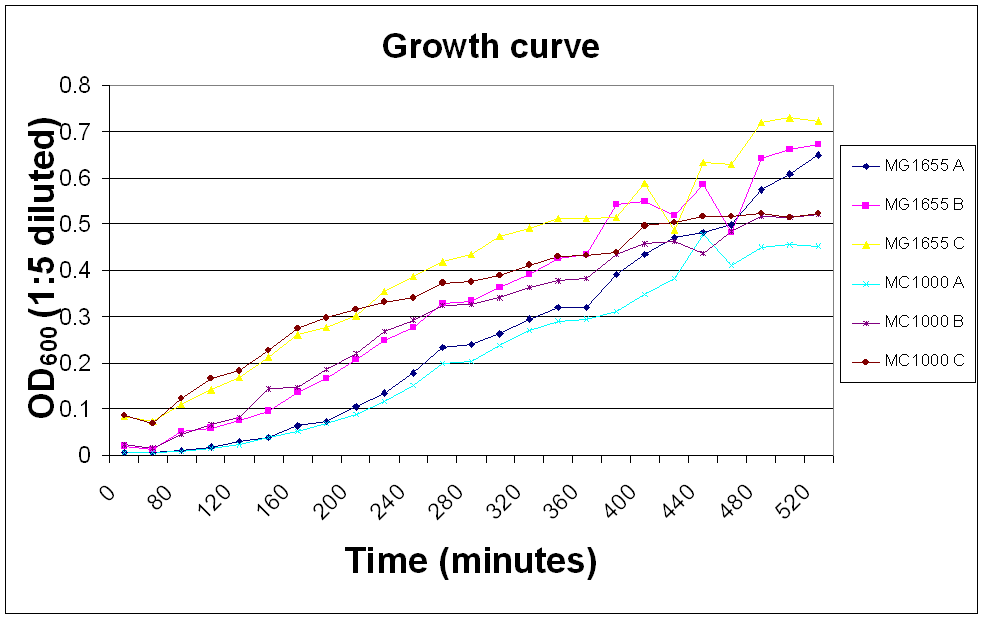
| + | The OD<sub>600</sub> of ''E. coli'' MC1000 and MG1655 was measured over a day so that we would know whether the bacteria were in their log phase in any experiments requiring innoculation of bacteria. | ||
| - | {| {{table}| align="center" |} {| border="1px" cellpadding="10" | + | A, B and C show 100, 500 and 200 µl innoculations of overnight culture in 50 ml LB broth, respectively. |
| + | |||
| + | |||
| + | {|align="center" {{table}| align="center" |} {| border="1px" cellpadding="10" | ||
| align="center" |'''Time (minutes)''' | | align="center" |'''Time (minutes)''' | ||
| align="center" |''''' | | align="center" |''''' | ||
| align="center" |''''' | | align="center" |''''' | ||
| align="center" |''''' | | align="center" |''''' | ||
| - | | align="center" |''' | + | | align="center" |'''OD<sub>600</sub> (1:5 diluted)''' |
| align="center" |''''' | | align="center" |''''' | ||
| align="center" |''''' | | align="center" |''''' | ||
| Line 86: | Line 91: | ||
|} | |} | ||
| + | |||
| + | {|align="center" | ||
| + | |||
| + | |[[Image:BCCS-080721-growth curve E. coli MC1000 and MG1655.PNG | 700px]] | ||
| + | |||
|} | |} | ||
| + | |||
| + | ==Swimming agar assay== | ||
| + | |||
| + | Chemotaxis experiment for MG1655 and MC1000 showed no real difference between the control (water) and the aspartate. This may have been due to higher agar concentration caused by an unusually large water loss in autoclaving, causing high concentrations of agar. | ||
| + | The experiment repetition was started today. | ||
| + | |||
| + | ==BioBrick Transformation== | ||
| + | |||
| + | The transformation of bacteria with BioBrick from paper was started. Therefore, a hole was punched out of the DNA spot of a [http://partsregistry.org/Part:BBa_E0240 GFP generator] and put in TE buffer (below) following protocol on iGEM sheet. | ||
| + | |||
| + | ==Miscellaneous== | ||
| + | |||
| + | DMSO stocks of ''E. coli'' MC1000 and MG1655 were made as back up in the -80<sup>o</sup>C freezer. | ||
Latest revision as of 14:01, 23 September 2008
Growth curve measurement
The OD600 of E. coli MC1000 and MG1655 was measured over a day so that we would know whether the bacteria were in their log phase in any experiments requiring innoculation of bacteria.
A, B and C show 100, 500 and 200 µl innoculations of overnight culture in 50 ml LB broth, respectively.
| Time (minutes) | OD600 (1:5 diluted) |
| |||||
| MG1655 | MC1000 | ||||||
| A | B | C | A | B | C | ||
| 0 | 0.0016 | 0.0042 | 0.017 | 0.0016 | 0.0044 | 0.0174 | |
| 60 | 0.008 | 0.013 | 0.073 | 0.006 | 0.017 | 0.068 | |
| 80 | 0.011 | 0.052 | 0.109 | 0.009 | 0.046 | 0.123 | |
| 100 | 0.019 | 0.059 | 0.142 | 0.017 | 0.067 | 0.167 | |
| 120 | 0.029 | 0.076 | 0.169 | 0.024 | 0.083 | 0.183 | |
| 140 | 0.039 | 0.095 | 0.213 | 0.039 | 0.144 | 0.226 | |
| 160 | 0.063 | 0.136 | 0.26 | 0.053 | 0.147 | 0.275 | |
| 180 | 0.074 | 0.166 | 0.276 | 0.069 | 0.186 | 0.298 | |
| 200 | 0.105 | 0.207 | 0.301 | 0.089 | 0.22 | 0.316 | |
| 220 | 0.134 | 0.25 | 0.355 | 0.116 | 0.268 | 0.331 | |
| 240 | 0.178 | 0.276 | 0.386 | 0.151 | 0.293 | 0.341 | |
| 260 | 0.233 | 0.329 | 0.419 | 0.198 | 0.325 | 0.372 | |
| 280 | 0.239 | 0.334 | 0.435 | 0.203 | 0.326 | 0.375 | |
| 300 | 0.263 | 0.363 | 0.473 | 0.238 | 0.34 | 0.388 | |
| 320 | 0.296 | 0.391 | 0.491 | 0.27 | 0.364 | 0.412 | |
| 340 | 0.319 | 0.425 | 0.511 | 0.29 | 0.378 | 0.429 | |
| 360 | 0.32 | 0.435 | 0.512 | 0.294 | 0.382 | 0.432 | |
| 380 | 0.392 | 0.542 | 0.514 | 0.311 | 0.434 | 0.438 | |
| 400 | 0.435 | 0.549 | 0.588 | 0.348 | 0.457 | 0.497 | |
| 420 | 0.47 | 0.52 | 0.487 | 0.382 | 0.462 | 0.504 | |
| 440 | 0.483 | 0.586 | 0.633 | 0.477 | 0.437 | 0.516 | |
| 460 | 0.498 | 0.482 | 0.629 | 0.411 | 0.484 | 0.517 | |
| 480 | 0.574 | 0.643 | 0.721 | 0.451 | 0.516 | 0.524 | |
| 500 | 0.607 | 0.66 | 0.73 | 0.455 | 0.514 | 0.515 | |
| 520 | 0.65 | 0.673 | 0.723 | 0.453 | 0.522 | 0.523 |
|
Swimming agar assay
Chemotaxis experiment for MG1655 and MC1000 showed no real difference between the control (water) and the aspartate. This may have been due to higher agar concentration caused by an unusually large water loss in autoclaving, causing high concentrations of agar. The experiment repetition was started today.
BioBrick Transformation
The transformation of bacteria with BioBrick from paper was started. Therefore, a hole was punched out of the DNA spot of a [http://partsregistry.org/Part:BBa_E0240 GFP generator] and put in TE buffer (below) following protocol on iGEM sheet.
Miscellaneous
DMSO stocks of E. coli MC1000 and MG1655 were made as back up in the -80oC freezer.
 "
"