Team:University of Lethbridge/Notebook/GeneralLabAugst
From 2008.igem.org
m (→August 5, 2008) |
Nathan.puhl (Talk | contribs) m |
||
| (7 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | ===August 01, 2008=== | ||
| + | ====Nathan Puhl, Nathan Phillips==== | ||
| + | |||
| + | '''Objective:''' Amplify more of the riboswitch using amplicons from the previous PCR | ||
| + | |||
| + | MasterMix: | ||
| + | 1x 4x | ||
| + | 5x buffer 5 uL 20 | ||
| + | 10 mM dNTP 0.5 uL 2 | ||
| + | RF primer 2.5 uL 10 | ||
| + | RR primer 2.5 uL 10 | ||
| + | Phusion 0.25 uL 1 | ||
| + | ddH2O 15.25 uL 61 | ||
| + | Template 1 uL of pTOPP, 1x, 1/10x riboswitch amplicon | ||
| + | |||
| + | Cycle Conditions: | ||
| + | 98 C - 3 min | ||
| + | 30x: 98 C - 10 sec | ||
| + | 55 C - 30 sec | ||
| + | 72 C - 15 sec | ||
| + | 72 C - 7 min | ||
| + | |||
| + | ===August 02, 2008=== | ||
| + | ====Nathan Puhl==== | ||
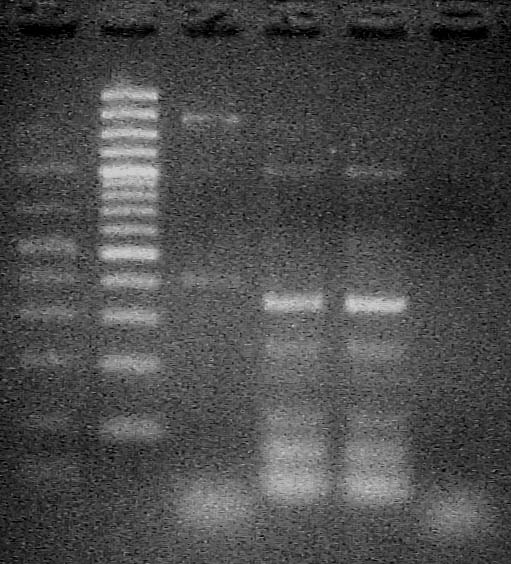
| + | Ran gel of PCR products from Aug. 01 with pTOPP template in lane 2, 1x, 1/10x amplicon template in lane 3 and 4 | ||
| + | |||
| + | [[Image:riboswitch-1.jpg]] | ||
| + | |||
===August 03, 2008=== | ===August 03, 2008=== | ||
====Nathan Phillips==== | ====Nathan Phillips==== | ||
| Line 15: | Line 43: | ||
===August 5, 2008=== | ===August 5, 2008=== | ||
====Nathan Puhl, Alix, Roxanne and highschool students==== | ====Nathan Puhl, Alix, Roxanne and highschool students==== | ||
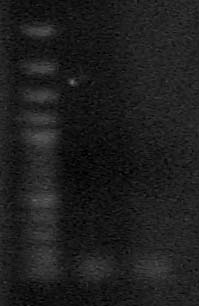
| + | '''Objective:''' Isolate 76bp band from non-specific amplified bands of riboswitch PCR | ||
| - | + | Using Qiagen MinElute Kit extracted two very, very similiarly sized bands after running on a 3.5% agarose gel. | |
| - | + | [[Image:purified riboswitch.jpg]] | |
| + | |||
| + | '''Objective:''' Cut out LacI gene and open DT vector for construction | ||
| + | |||
| + | Restriction Digest of BBa_C0012 (LacI) and BBa_C0012 (Double Terminator) | ||
| + | |||
| + | LacI - EcoR I + Spe I | ||
| + | |||
| + | DT - EcoR I + Xba I | ||
| + | |||
| + | Overnight incubation at 37 C followed by 65 C for 15 min (enzyme deactivation) | ||
| + | |||
| + | Reaction Mixture: | ||
| + | -5 uL template | ||
| + | -5 uL NEB 2 buffer | ||
| + | -0.5 uL BSA | ||
| + | -1 uL restriction enzyme #1 | ||
| + | -1 uL restriction enzyme #2 | ||
| + | -37.5 uL ddH2O | ||
===August 6, 2008=== | ===August 6, 2008=== | ||
====Nathan Puhl, Roxanne, Alix==== | ====Nathan Puhl, Roxanne, Alix==== | ||
| - | + | '''Objective:''' Gel extract digested biobricks to clean for ligation reaction | |
| + | |||
| + | Qiagen MinElute Kit was used to gel purify the digested LacI and DT biobricks. | ||
| + | |||
| + | ===August 7, 2008=== | ||
| + | ====Nathan Puhl, Roxanne, Peter==== | ||
| + | '''Objective:''' Amplify riboswitch with A overhangs for TA cloning | ||
| + | |||
| + | Set up PCR using purified riboswitch amplicon from Aug. 05 (1 uL of template) | ||
| + | |||
| + | MasterMix: | ||
| + | 1x 3x | ||
| + | 10x buffer 5 uL 15 | ||
| + | 10 mM dNTP 1 uL 3 | ||
| + | 50 mM Mg2+ 1.5 4.5 | ||
| + | RF primer 1 uL 3 | ||
| + | RR primer 1 uL 3 | ||
| + | Platinum taq 0.2 uL 0.6 | ||
| + | ddH2O 39.3 uL 117.9 | ||
| + | Template 1 uL of riboswitch amplicon | ||
| - | - | + | Cycle Conditions: |
| + | 94 C - 2 min | ||
| + | 30x: 94 C - 30 sec | ||
| + | 53 C - 30 sec | ||
| + | 72 C - 30 sec | ||
Latest revision as of 01:41, 8 August 2008
Contents |
August 01, 2008
Nathan Puhl, Nathan Phillips
Objective: Amplify more of the riboswitch using amplicons from the previous PCR
MasterMix:
1x 4x
5x buffer 5 uL 20
10 mM dNTP 0.5 uL 2
RF primer 2.5 uL 10
RR primer 2.5 uL 10
Phusion 0.25 uL 1
ddH2O 15.25 uL 61
Template 1 uL of pTOPP, 1x, 1/10x riboswitch amplicon
Cycle Conditions:
98 C - 3 min
30x: 98 C - 10 sec
55 C - 30 sec
72 C - 15 sec
72 C - 7 min
August 02, 2008
Nathan Puhl
Ran gel of PCR products from Aug. 01 with pTOPP template in lane 2, 1x, 1/10x amplicon template in lane 3 and 4
August 03, 2008
Nathan Phillips
Tranformation of pUC19:
1. Thaw E.coli DH5a cells on ice 2. Add pUC19 DNA, pipette gently to mix (1μl of plasmid) 3. Let sit for 30 minutes on ice 4. Incubate cells for 30 seconds at 42oC 5. Incubate cells on ice for 2 min 6. Add 1 mL SOC at room temp 7. Incubate for 1 hour at 37oC on shaker 8. Spread 100-300 μl onto a plate made with appropriate antibiotic 9. Grow overnight at 37 °C
August 5, 2008
Nathan Puhl, Alix, Roxanne and highschool students
Objective: Isolate 76bp band from non-specific amplified bands of riboswitch PCR
Using Qiagen MinElute Kit extracted two very, very similiarly sized bands after running on a 3.5% agarose gel.
Objective: Cut out LacI gene and open DT vector for construction
Restriction Digest of BBa_C0012 (LacI) and BBa_C0012 (Double Terminator)
LacI - EcoR I + Spe I
DT - EcoR I + Xba I
Overnight incubation at 37 C followed by 65 C for 15 min (enzyme deactivation)
Reaction Mixture:
-5 uL template -5 uL NEB 2 buffer -0.5 uL BSA -1 uL restriction enzyme #1 -1 uL restriction enzyme #2 -37.5 uL ddH2O
August 6, 2008
Nathan Puhl, Roxanne, Alix
Objective: Gel extract digested biobricks to clean for ligation reaction
Qiagen MinElute Kit was used to gel purify the digested LacI and DT biobricks.
August 7, 2008
Nathan Puhl, Roxanne, Peter
Objective: Amplify riboswitch with A overhangs for TA cloning
Set up PCR using purified riboswitch amplicon from Aug. 05 (1 uL of template)
MasterMix:
1x 3x
10x buffer 5 uL 15
10 mM dNTP 1 uL 3
50 mM Mg2+ 1.5 4.5
RF primer 1 uL 3
RR primer 1 uL 3
Platinum taq 0.2 uL 0.6
ddH2O 39.3 uL 117.9
Template 1 uL of riboswitch amplicon
Cycle Conditions:
94 C - 2 min
30x: 94 C - 30 sec
53 C - 30 sec
72 C - 30 sec
 "
"