Team:Caltech/Project/Vitamins
From 2008.igem.org
(→Modifications to System Design) |
|||
| (71 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | <div | + | {{Caltech_iGEM_08| |
| - | < | + | Content= |
| - | + | ||
| - | + | <div style="font-size:18pt;"> | |
| - | </div> | + | <font face="verdana" style="color:#BB4400">''In vivo'' Folate Production</font></div> |
| - | < | + | <br> |
| - | + | ||
| - | + | ||
| - | + | ||
==Background Information on Folate== | ==Background Information on Folate== | ||
| - | [[Image:THF_structure.jpg|thumb|left|The structure of tetrahydrofolate | + | [[Image:THF_structure.jpg|thumb|left|The structure of tetrahydrofolate.]] |
| - | + | ||
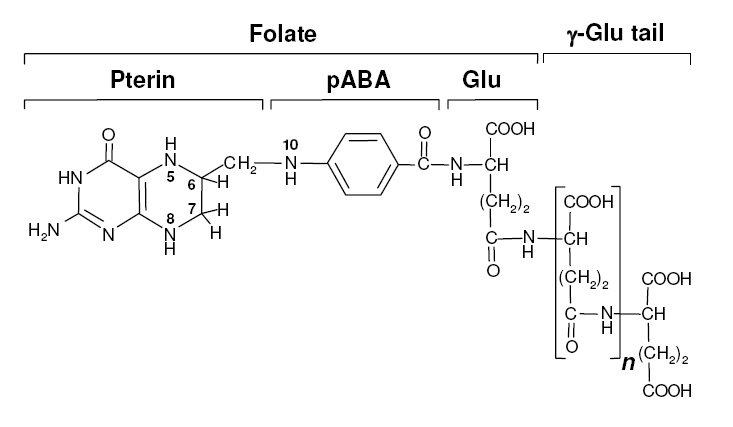
| - | + | Folate, the generic term for the various forms of Vitamin B9, is an essential vitamin involved in single-carbon transfer reactions, which are important for many pathways including amino acid synthesis. Folate deficiencies in women can result in birth defects such as neural tube defects and other spinal cord malformations. As important as folate is, humans are unable to produce folate, and so must obtain it from eating foods such as green leafy vegetables or folate-fortified cereals. An engineered strain of bacteria that would constantly release folate into the gut would reduce the need to fortify breads and cereals with folate, as well as reduce folate-related birth defects in regions with little access to folate-containing foods. In addition to all the reasons stated above, folate is an ideal vitamin to be produced in the gut because, unlike many other vitamins, it has been shown to be absorbed in physiologically relevant quantities in the large intestine<sup>1, 2</sup>. | |
| + | Structurally, folate consists of three main parts: pteridine (derived from GTP), p-aminobenzoic acid (PABA, derived from chorismate), and a poly-glutamyl tail (derived from linking glutamate). | ||
==Folate Biosynthesis Pathway== | ==Folate Biosynthesis Pathway== | ||
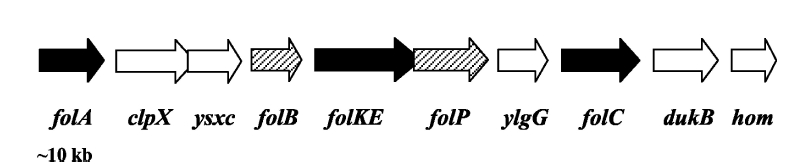
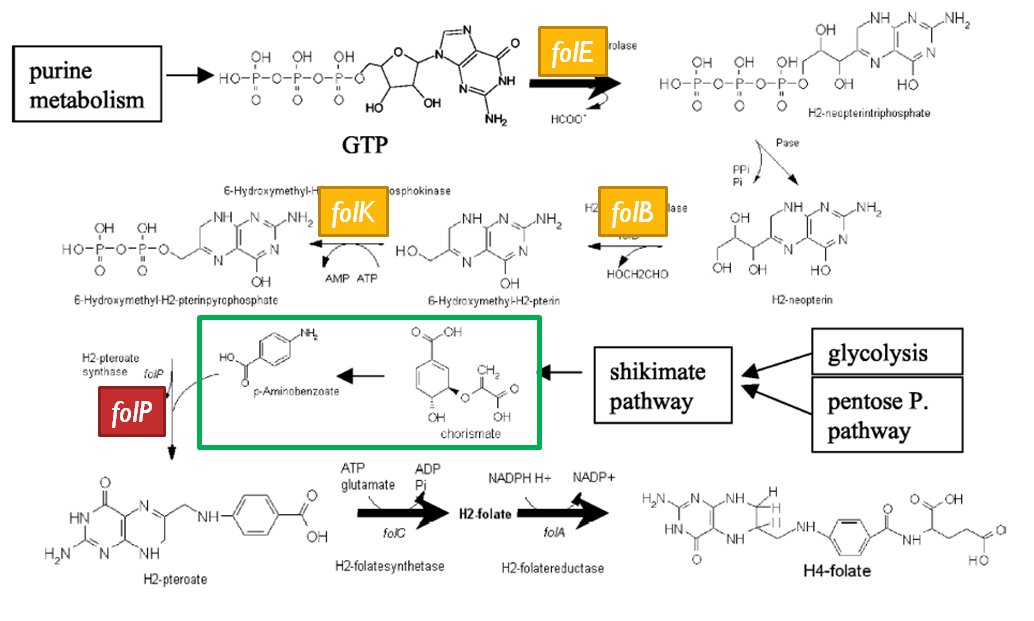
| - | [[Image:folate_gene_cluster.jpg |frame|center|50px|The folate gene cluster from ''L.lactis''. Black arrows represent genes which have been tested in metabolic engineering studies, shaded arrows represent genes involved in folate biosynthesis, and white arrows represent genes not involved in folate synthesis | + | [[Image:folate_gene_cluster.jpg |frame|center|50px|The folate gene cluster from ''L.lactis''. Black arrows represent genes which have been tested in metabolic engineering studies, shaded arrows represent genes involved in folate biosynthesis, and white arrows represent genes not involved in folate synthesis<sup>3</sup>.]] |
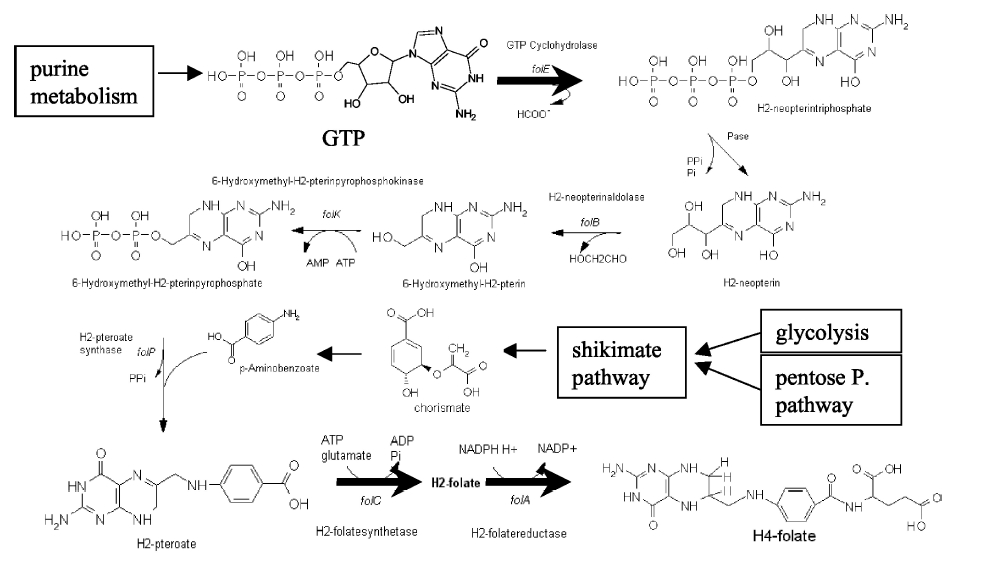
| - | Although folate is naturally produced in ''E.coli'', the folate biosynthesis pathway in the bacteria ''Lactococcus lactis'' has been more heavily characterized and studied. There are six major | + | Although folate is naturally produced in ''E. coli'', the folate biosynthesis pathway in the bacteria ''Lactococcus lactis'' has been more heavily characterized and studied. In previous studies, this folate gene cluster has been successfully transformed into the folate-consuming bacteria ''L. gasseri'', turning the bacteria into folate-producers<sup>4</sup>. Using the folate gene cluster from ''L. lactis'' also offers the additional benefit of removing the operon from its natural regulatory context. There are six major enzymatic activities involved in folate synthesis, which, in ''L. lactis'', are contained in five genes: ''folB'', ''folKE'', ''folP'', ''folC'', and ''folA''<sup>1</sup>. The first four, which we have chosen to focus on, are located in a gene cluster approximately 4.4kb long. We have chosen not to test overexpression of ''folA'' because it encodes for an enzyme which turns one form of folate (dihydrofolate) into another form of folate (tetrahydrofolate). Since our assay would detect both types of folate as part of the total folate production, ''folA'' was not a prime target for overexpression of folate. |
| - | + | ||
| - | + | ||
| - | + | ||
| + | [[Image:Llactis_folate_synthesis.jpg|thumb|left|The folate biosynthesis pathway from ''L.lactis''<sup>3</sup>.]] | ||
| + | Our strategy is to clone the entire folate operon from the ''L.lactis'' genome and to transform the entire operon into ''E.coli''. However, because we are unsure of whether or not the ribosomal binding sites (RBS) within the ''L.lactis'' operon would work in ''E.coli'', we are also going to unpack the operon by cloning each of the four genes individually, placing them behind ''E.coli'' RBSs, and then recombine them into a single empty BioBricks™ plasmid. In addition to the main folate operon, we will also be experimenting with overexpression of the para-aminobenzoic acid (pABA) synthesis pathway from chorismate. Wegkamp ''et al.'' have shown that in order to increase overall total levels of folate, both the pABA synthesis genes and certain folate production genes need to be overexpressed<sup>5</sup>. The pABA pathway involves three enzymatic activities, ''pabA'', ''pabB'', and ''pabC'' – though in ''L.lactis'', ''pabB'' is actually a bifunctional enzyme containing the activities for both ''pabB'' and ''pabC''<sup>5</sup>. | ||
| + | {{clear}} | ||
==System Design== | ==System Design== | ||
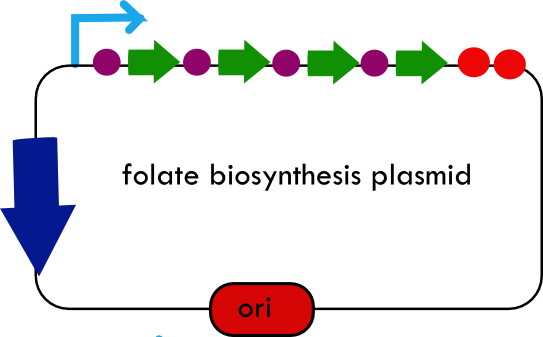
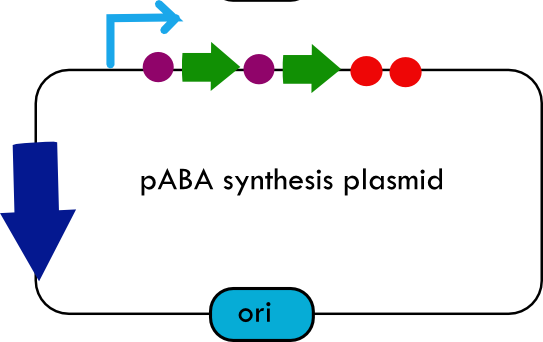
[[Image:systemdesign_folate.png|thumb|right|Final folate biosynthesis plasmid]] | [[Image:systemdesign_folate.png|thumb|right|Final folate biosynthesis plasmid]] | ||
| - | [[Image:systemdesign_paba.png|thumb|right|Final | + | [[Image:systemdesign_paba.png|thumb|right|Final PABA biosynthesis plasmid]] |
| - | + | ||
| - | + | ||
| + | The overall system design for testing folate production in ''E. coli'' is to construct several plasmids: one for each individual gene, one for the folate biosynthesis pathway, and one for the PABA synthesis pathway. Each gene, or gene cluster, would be cloned in an inducible-copy plasmid, which would be low copy by default, but can be switched to high copy via the addition of Isopropyl β-D-1-thiogalactopyranoside (IPTG)to the media. This will allow us to test overexpression of each plasmid separately. In addition, each plasmid contains a constitutive promoter, to ensure the constant production of folate. | ||
| + | {{clear}} | ||
==Folate Detection Methods== | ==Folate Detection Methods== | ||
| + | [[Image:Lrham.jpg| thumb| left| Image of ''Lactobacillus rhamnosus.'' Itself a probiotic strain, ''L. rhamnosus'' is commonly used in yogurt [http://www.yaklasansaat.com/resimler/2008haberresimleri/haber_subat_resim/subat19_lactobacillus_rhamnosus.jpg][http://en.wikipedia.org/wiki/Lactobacillus_rhamnosus].]] | ||
| + | We measured folate production, and thus the relative success of our engineering efforts, via a microbiological assay involving the folate-dependent strain ''Lactobacillus rhamnosus'' (ATCC 7469)<sup>6</sup>. The assay uses growth of the folate-dependent strain ''L. rhamnosus'' as an indicator of folate concentrations in the sample. The assay media specifically lacks folate, so folate becomes the limiting factor in the growth of ''L. rhamnosus'', with the only possible source being the bacterial lysates of the engineered ''E. coli''. In order to quantify the relative growth of the folate-dependent strain ''L. rhamnosus'', a standard growth curve must first be characterized using known quantities of folic acid in assay media. Once the standard curve has been established, then experimental growth levels, as quantified by spectrophotometry, can be interpolated. Protocols are based off of BD Biosciences Folate Assay Medium datasheet<sup>7</sup>. Growth was measured by taking the OD at 546nm. | ||
| - | + | Further details are available on the [[Team:Caltech/Protocols/Folate assay|<font style="color:#BB4400">folate microbiological assay protocol</font>]] page. | |
| + | {{clear}} | ||
| + | ==pABA Detection Methods== | ||
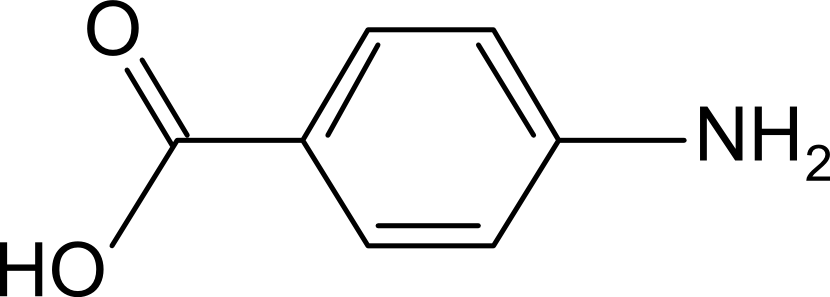
| + | [[Image:PABA-chem.png|thumb|left|para-Aminobenzoic Acid [http://en.wikipedia.org/wiki/4-Aminobenzoic_acid]]] | ||
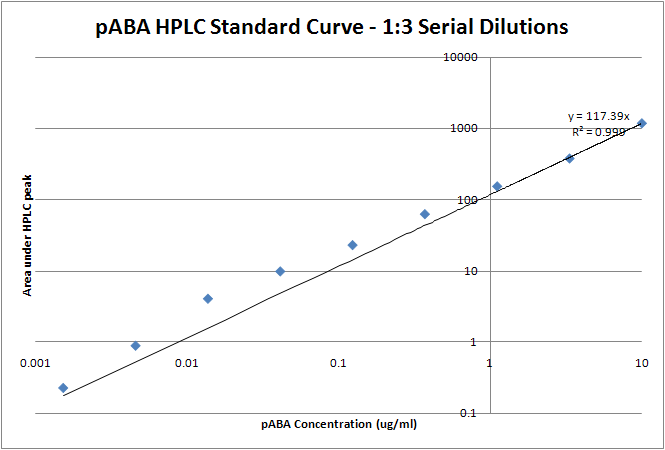
| + | para-Aminobenzoic Acid (PABA) can be detected using high performance liquid chromatography (HPLC)<sup>8</sup>. Using a 14 minute protocol, we were able to detect PABA peaks coming off a C18 column at 4.9 min. A standard curve was made by running a series of 1:3 PABA dilutions starting at a 10μg/ml concentration. The PABA for the standard curve was spiked into wild type ''E. coli'' cell lysate, which by itself did not show any detectable PABA peaks. Samples were run using the same HPLC protocol as the standards, and both lysate and supernatant were tested for PABA peaks. | ||
| - | + | Again, details on the PABA detection method can be found on the [[Team:Caltech/Protocols/PABA HPLC assay|<font style="color:#BB4400">para-aminobenzoic acid (pABA) HPLC protocol</font>]] page. | |
| - | + | ||
| - | [[Team:Caltech/Protocols/PABA HPLC assay| para-aminobenzoic acid (pABA) HPLC protocol]] | + | |
==Results== | ==Results== | ||
===Modifications to System Design=== | ===Modifications to System Design=== | ||
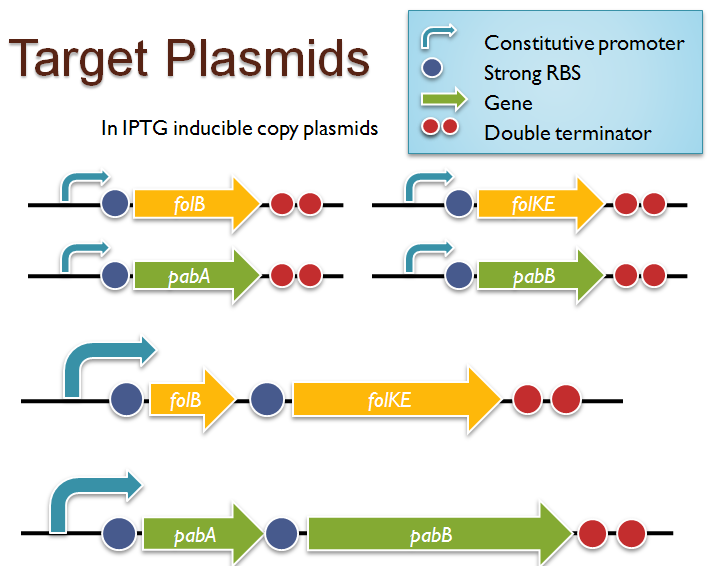
| + | [[Image:Target_constructs.png | thumb | right | Target constructs for folate biosynthesis and pABA synthesis gene overexpression. We were able to successfully clone ''folB'' in an inducible-copy plasmid and ''folKE'' and ''pabA'', in high-copy plasmids. Unfortunately, we were unable to complete the ''folBKE'', ''pabB'', or ''pabA + pabB'' constructs.]] | ||
| + | We were able to successfully extract and clone the following three genes: ''folB'', ''folKE'', and ''pabA''. We aimed to create individual constructs of each gene in the IPTG inducible-copy plasmid pSB2K3, as well as constructs with the two folate genes combined and with the two PABA synthesis genes combined. However, we had issues cloning our genes into the pSB2K3 + B0015 terminator construct, and so switched to completing the constructs in the high copy plasmid pSB1AK3 + B0015 terminator. | ||
| - | + | Our final constructs, shown on the right, are ''folB'' in pSB2K3, and ''folKE'' and ''pabA'' in pSB1AK3. We were unable to sequence confirm our ''folBKE'' and ''pabB'' constructs, and so unfortunately could not include their data. Previous studies on folate overexpression have shown that both folate synthesis and PABA synthesis genes need to be overexpressed simultaneously in order to increase total folate levels<sup>3</sup>. Therefore, tests on ''folB'' and ''folKE'' were done with and without the addition of PABA during the ''E. coli'' inoculation; detection of higher folate levels on addition of PABA would indicate PABA as a limiting factor for folate production. | |
| - | + | We were unable to use PCR to extract several of the target genes: the entire folate gene cluster, ''folP'', and ''folC''. After analyzing the sequencing results of our other folate biosynthesis genes, we realized that the genetic sequences had several point mutations which were not `stop' codons. This indicated that we had a homologous, but not identical, genomic DNA than the one that we has used for PCR primer design. We believe that this homologous sequence could have resulted in too many differences between the primer and the genomic DNA, resulting in little to no binding. This may explain why we were unable to successfully extract ''folP'', ''folC'', and subsequently, the entire folate gene cluster (since ''folP'' and ''folC'' are the last two genes in the cluster) from the genomic DNA of ''L. lactis''. The revised target constructs are shown . | |
| - | |||
| - | ==Relevant | + | ===Folate Assay Results for ''folB'' AND ''folKE''=== |
| - | + | ||
| - | { | + | The experimental setup included running a standard curve with known amounts of folic acid (0-10ng) simultaneously with the samples, in order to have the same basis for comparison. The hope was that the standard curve growth OD values would correspond with known concentrations, and so sample concentrations could be interpolated based upon the standard. ''folKE'', which was cloned into a constitutive high-copy plasmid, was tested with and without the addition of 500 ng of PABA during inoculation. ''folB'', which is in an inducible copy plasmid, it was tested induced to high-copy as well as uninduced low-copy, with and without 500 ng of PABA during inoculation. We assayed both the supernatant and the cell lysate, though only the supernatant had measurable results. |
| - | + | ||
| + | The growth data for the samples are very encouraging since the relative folate levels match what we would expect. We see that the addition of 500 ng of PABA during inoculation dramatically increases overall folate levels for ''folKE'' relative to the samples without PABA. Furthermore, adding PABA to the wild type controls did not affect growth at all, suggesting that the assay bacteria ''L.rhamnosus'' was not metabolizing the extra PABA. The extremely high OD values for all the samples were possibly the result of not completely washing out all of the culture media prior to adding the ''L.rhamnosus'' to the assay samples. | ||
| + | |||
| + | <gallery widths="375px" heights="250px" > | ||
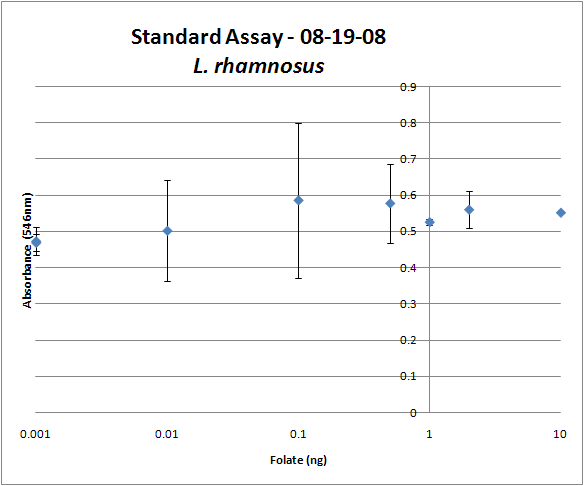
| + | Image:08-19-08 Folate Assay Std Curve Avg.png|Test #1: Folate Standard Curve from 0-10ng. This standard curve is pretty much useless. | ||
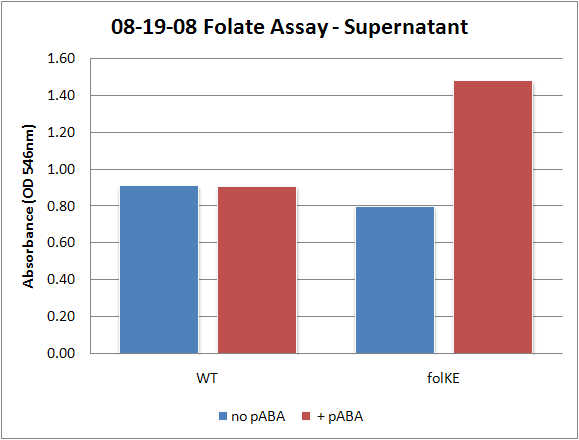
| + | Image:08-19-08 Folate Assay supernatant.png|Test #1: Relative folate levels for ''folKE'' compared to ''E. coli'' wild type. Here we see encouraging confirmation of all the expected trends - the addition of PABA during inoculation increases total folate levels but only in the samples with overexpression of our overexpressed folate constructs. | ||
| + | </gallery> | ||
| + | When we repeated the assay in duplicate, this time on ''folB'' as well, we were able to observe the same trends in growth with and without the addition of PABA. Again, we see that for ''folKE'', the addition of PABA does increase total folate levels, though not as dramatically as before. The rampant growth of the wild type control is disconcerting, but wild type folate production again appears to be unaffected by the addition of PABA. Furthermore, folate levels for ''folKE'' are still higher than the control where ''L.rhamnosus'' was added to only media without supernatant. | ||
| + | <gallery widths="375px" heights="250px" > | ||
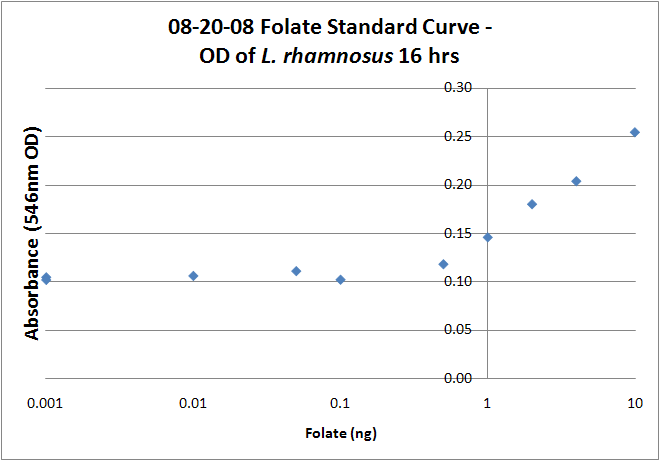
| + | Image:08-20-08 Folate Assay Std 16 hrs.png|Test #2: Folate Standard Assay.A beautiful linear range between .1 and 10 ng can be seen, but overall growth does not correspond to sample growth. Could we be saturating the standard? | ||
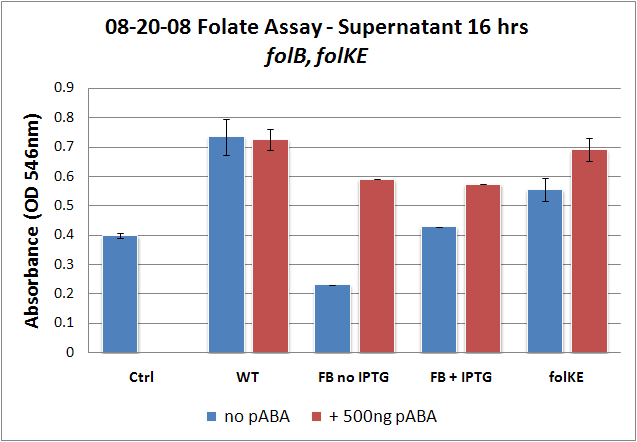
| + | Image:08-20-08 Folate Assay supernatant 16 hrs folKE, BKE.png|Test #2: Folate Assay Results with ''folKE'' and ''folB''. Although the wild type growth is mystifying, the folate levels for ''folKE'' exhibit the same trends as before, where increased folate is observed with the addition of PABA. ''FolB'' was tested in low (no IPTG) and high (with IPTG) copy since it is in an inducible-copy plasmid. In the folB data, induced high copy without PABA shows higher growth than low copy without PABA, and the addition of PABA shows the highest growth of all. It is interesting to note that that the two +PABA levels for ''folB'' appear to be the same. | ||
| + | </gallery> | ||
| + | |||
| + | [[Image:bottleneck.png|thumb|right|Increasing the flux of both pathways upstream of pABA integration into the pterin (derived from GTP) component of folate may have created a bottleneck. Unfortunately we were unable to test this hypothesis since we could not overexpress ''folP''<sup>3</sup>]] | ||
| + | And what of ''folB''? Recall that ''folB'' was the only gene to be successfully cloned into an inducible-copy plasmid, and so it was tested both induced and uninduced. The folate levels in the induced sample (high-copy) are higher than in the uninduced (low-copy) sample, which is consistent with what we would expect. The addition of PABA to both induced and uninduced increases relative levels of folate, which is also consistent. However, it is interesting to note that folate levels for the +PABA samples are the same for induced and uninduced. Of course, given the small sample size, this could just be due to variability, but it could also suggest that folate production reached an upper limit. This explanation seems even more likely if we reconsider the folate biosynthesis pathway, and we see that ''folB'' and ''folKE'' are both located upstream of actual integration of PABA into the molecule, accomplished by ''folP''. It is very possible that we are increasing the flux of both pathways going into the ''folP'' junction, but as we were unable to overexpress ''folP'' as well, we may have created a bottleneck. | ||
| + | {{clear}} | ||
| + | |||
| + | ===para-Aminobenzoic Acid (pABA) HPLC Assay Results for ''pabA''=== | ||
| + | <gallery widths="375px" heights="250px" > | ||
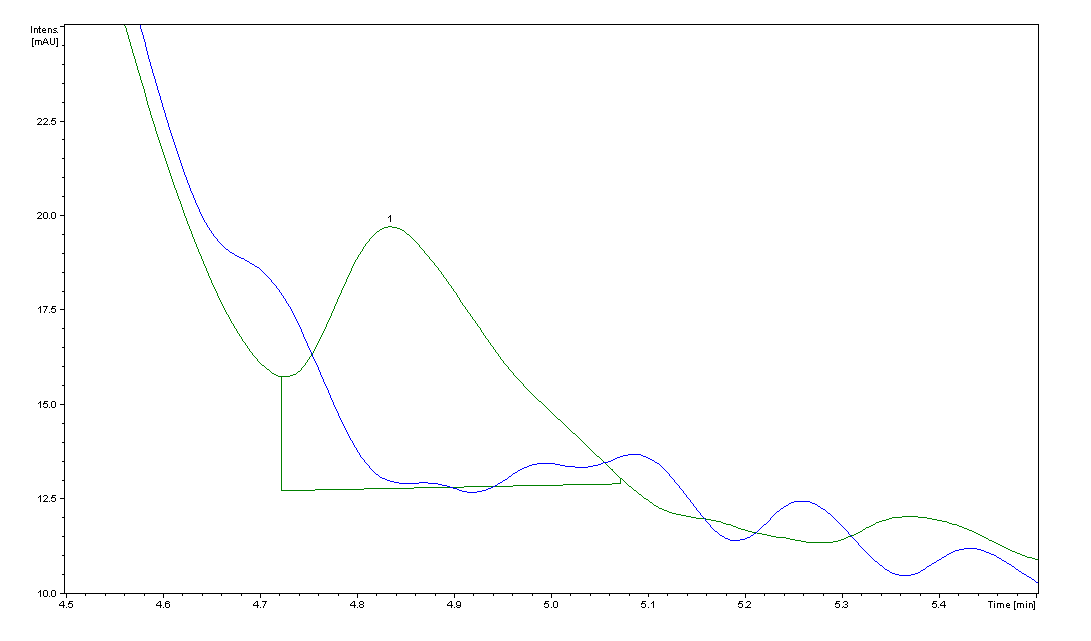
| + | Image:pABA.png|HPLC peaks for the ''pabA'' sample. The blue trace is the control cell supernatant, which has no peak at 4.9 min, the elution time we found for PABA. The area for the ''pabA'' peak, shown in green, is 82.55. | ||
| + | Image:pABA trendline.png| Standard Curve with linear trendline (y = 117.39x). Using this linear approximation, the concentration of pABA is 703 ng/mL for ''pabA'' overexpression. | ||
| + | </gallery> | ||
| + | |||
| + | Using high performance liquid chromatography (HPLC) we were able to detect PABA peaks from overexpression of ''pabA'' compared to a control sample of wild type supernatant. Compared to the peak areas of the standard curve, we were able to use a linear approximation to determine the concentration of PABA to be 703 ng/mL for ''pabA'' overexpression. | ||
| + | |||
| + | ===Conclusions and Future Work=== | ||
| + | |||
| + | We have shown, through very preliminary tests, the feasibility of overproduction of folate in ''E. coli'' using genes originally derived from ''L. lactis''. Our data have confirmed previous studies in the necessity of overexpressing both folate and PABA synthesis genes, and we have shown that folate appears to be mostly present in the supernatant. Our data, again confirming previous results<sup>9</sup>, also suggest that overexpression of ''folKE'' is the most effective, definitely more effective than ''folB''. | ||
| + | |||
| + | We have also shown that it is possible to overexpress para-aminobenzoic acid production in ''E. coli'' and that overexpression of ''pabA'' increases total levels of para-aminobenzoic acid (PABA). | ||
| + | |||
| + | Further work on this project would include repeating the folate assay to generate more data, perfecting the assay in order to quantify folate levels with the standard curve, making the ''pabA'' + ''pabB'' and ''folB'' + ''folKE'' constructs, making a ''folB'' + ''folKE'' + ''pabA'' + ''pabB'' construct, and extracting and cloning ''folP'' from the ''L. lactis'' genome. | ||
| + | |||
| + | ==Relevant Parts== | ||
| + | ==Basic Parts== | ||
| + | {{{!}} border="1" | ||
| + | {{!}}+ Basic Parts (Extracted from ''L.lactis subspecies IL1403'' genome) | ||
! Part Name !! Registry # !! Description !! Cloned? !! Sequence confirmed? | ! Part Name !! Registry # !! Description !! Cloned? !! Sequence confirmed? | ||
| - | + | {{!}}- | |
! Entire folate synthesis operon | ! Entire folate synthesis operon | ||
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137002 BBa_K137002]{{!}}{{!}} Includes folB+folKE+folP+ylgG+folC {{!}}{{!}} NO {{!}}{{!}} NO | |
| - | + | {{!}}- | |
! folB | ! folB | ||
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137009 BBa_K137009]{{!}}{{!}} dihydroneopterin aldolase {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
! folKE | ! folKE | ||
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137011 BBa_K137011]{{!}}{{!}} fusion gene: GTP cyclohydrolase & 2-amino-4-hydroxy-6- hydroxymethyldihydropteridine pyrophosphokinase {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
! folP | ! folP | ||
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137012 BBa_K137012]{{!}}{{!}} Dihydropteroate synthase {{!}}{{!}} NO{{!}}{{!}} NO | |
| - | + | {{!}}- | |
! folC | ! folC | ||
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137013 BBa_K137013]{{!}}{{!}} folate synthetase/polyglutamyl folate synthetase {{!}}{{!}} NO{{!}}{{!}} NO | |
| - | + | {{!}}- | |
! pabA | ! pabA | ||
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137005 BBa_K137005]{{!}}{{!}} para-aminobenzoate synthetase component II {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
! pabB | ! pabB | ||
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137006 BBa_K137006]{{!}}{{!}} para-aminobenzoate synthetase component I {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | + | {{!}}} | |
| - | + | ==Construction Intermediates== | |
| - | { | + | {{{!}} border="1" |
| - | + | {{!}}+ Construction Intermediates: Adding B0034 (strong RBS) before each individual gene | |
! Part Name !! Registry # !! Description !! Cloned? !! Sequence confirmed? | ! Part Name !! Registry # !! Description !! Cloned? !! Sequence confirmed? | ||
| - | + | {{!}}- | |
| - | ! folB + | + | ! folB + B0034 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S03957 BBa_S03957]{{!}}{{!}} RBS + dihydroneopterin aldolase {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! folKE + | + | ! folKE + B0034 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S03958 BBa_S03958]{{!}}{{!}} RBS + fusion gene: GTP cyclohydrolase & 2-amino-4-hydroxy-6- hydroxymethyldihydropteridine pyrophosphokinase {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! folP + | + | ! folP + B0034 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S03959 BBa_S03959]{{!}}{{!}} RBS + Dihydropteroate synthase {{!}}{{!}} NO{{!}}{{!}} NO | |
| - | + | {{!}}- | |
| - | ! folC + | + | ! folC + B0034 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S03960 BBa_S03960]{{!}}{{!}} RBS + folate synthetase/polyglutamyl folate synthetase {{!}}{{!}} NO{{!}}{{!}} NO | |
| - | + | {{!}}- | |
| - | ! pabA + | + | ! pabA + B0034 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S03976 BBa_S03976]{{!}}{{!}} RBS + para-aminobenzoate synthetase component II {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! pabB + | + | ! pabB + B0034 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S03977 BBa_S03977]{{!}}{{!}} RBS + para-aminobenzoate synthetase component I {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! | + | ! B0034 + folB + B0034 + folKE |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S03961 BBa_S03961]{{!}}{{!}} Combining folB (with RBS) + folKE (with RBS) {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | + | {{!}}} | |
| - | { | + | {{{!}} border="1" |
| - | + | {{!}}+ Construction Intermediates: Adding J23100 (constitutive promoter) to each gene (with RBS already) | |
! Part Name !! Registry # !! Description !! Cloned? !! Sequence confirmed? | ! Part Name !! Registry # !! Description !! Cloned? !! Sequence confirmed? | ||
| - | + | {{!}}- | |
| - | ! folB + | + | ! folB + J23100 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S04032 BBa_S04032]{{!}}{{!}} Promoter(j23100) + RBS(b0034) + dihydroneopterin aldolase {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! folKE + | + | ! folKE + J23100 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S04033 BBa_S04033]{{!}}{{!}} Promoter + RBS + fusion gene: GTP cyclohydrolase & 2-amino-4-hydroxy-6- hydroxymethyldihydropteridine pyrophosphokinase {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! pabA + | + | ! pabA + J23100 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S04034 BBa_S04034]{{!}}{{!}} Promoter + RBS + para-aminobenzoate synthetase component II {{!}}{{!}} YES{{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! folBKE + | + | ! folBKE + J23100 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S04035 BBa_S04035]{{!}}{{!}} Promoter + folB (with RBS) + folKE (with RBS) {{!}}{{!}} YES {{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! pabB + | + | ! pabB + J23100 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S04039 BBa_S04039]{{!}}{{!}} Promoter + pabB {{!}}{{!}} YES {{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! pabB + | + | ! pabB + B0015 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_S04038 BBa_S04038]{{!}}{{!}} pabB + b0015 terminator {{!}}{{!}} MAYBE {{!}}{{!}} NO | |
| - | + | {{!}}- | |
| - | + | {{!}}} | |
===Composite Parts=== | ===Composite Parts=== | ||
| - | { | + | {{{!}} border="1" |
| - | + | {{!}}+ Composite Parts: Adding B0015 (double terminator) to complete constructs with promoter + RBS already | |
! Part Name !! Registry # !! Description !! Cloned? !! Sequence confirmed? | ! Part Name !! Registry # !! Description !! Cloned? !! Sequence confirmed? | ||
| - | + | {{!}}- | |
| - | ! folB + | + | ! folB + B0015 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137053 BBa_K137053]{{!}}{{!}} Promoter(j23100) + RBS(b0034) + folB (dihydroneopterin aldolase) + double terminator (b0015) {{!}}{{!}} YES {{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! folKE + | + | ! folKE + B0015 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137054 BBa_K137054]{{!}}{{!}} Promoter + RBS + folKE (fusion gene: GTP cyclohydrolase & 2-amino-4-hydroxy-6- hydroxymethyldihydropteridine pyrophosphokinase) + double terminator (b0015) {{!}}{{!}} YES {{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! pabA + | + | ! pabA + B0015 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137055 BBa_K137055]{{!}}{{!}} Promoter + RBS + pabA (para-aminobenzoate synthetase component II) {{!}}{{!}} YES {{!}}{{!}} YES | |
| - | + | {{!}}- | |
| - | ! pabB + | + | ! pabB + B0015 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137109 BBa_K137109]{{!}}{{!}} Promoter + RBS + pabB (para-aminobenzoate synthetase component I) {{!}}{{!}} YES {{!}}{{!}} NO | |
| - | + | {{!}}- | |
| - | ! folBKE + | + | ! folBKE + B0015 |
| - | + | {{!}} [http://partsregistry.org/Part:BBa_K137056 BBa_K137056]{{!}}{{!}} Promoter + RBS + folB + folKE + b0015 {{!}}{{!}} YES {{!}}{{!}} NO | |
| - | + | {{!}}- | |
| - | + | {{!}}} | |
==References== | ==References== | ||
| - | + | [1] Asrar FM and O’Connor DL. '''Bacterially synthesized folate and supplemental folic acid are absorbed across the large intestine of piglets.''' J Nutr Biochem 2005 Oct; 16(10) 587-93. | |
| - | + | ||
| - | + | [2] Camilo E, Zimmerman J, Mason JB, Golner B, Russell R, Selhub J, and Rosenberg IH. '''Folate synthesized by bacteria in the human upper small intestine is assimilated by the host.''' Gastroenterology 1996 Apr; 110(4) 991-8. | |
| - | + | ||
| - | + | [3] Sybesma W, Starrenburg M, Kleerebezem M, Mierau I, de Vos WM, and Hugenholtz J. '''Increased production of folate by metabolic engineering of Lactococcus lactis.''' Appl Environ Microbiol 2003 Jun; 69(6) 3069-76. | |
| - | + | ||
| - | + | [4] Wegkamp A, Starrenburg M, de Vos WM, Hugenholtz J, and Sybesma W. '''Transformation of folate-consuming Lactobacillus gasseri into a folate producer.''' Appl Environ Microbiol 2004 May;70(5) 3146-8. | |
| - | + | ||
| - | + | [5] Wegkamp A, van Oorschot W, de Vos WM, and Smid EJ. '''Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis.''' Appl Environ Microbiol 2007 Apr; 73(8) 2673-81. | |
| - | + | ||
| - | + | [6] Horne DW and Patterson D. '''Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates.''' Clin Chem 1988 Nov; 34(11) 2357-9. | |
| - | + | ||
| - | + | [7] BD Biosciences. '''Folic Acid Assay Medium.''' [http://www.bd.com/ds/technicalCenter/inserts/Folic_Acid_Assay_Medium.pdf] | |
| - | + | ||
| - | + | [8] Zhang GF, Mortier KA, Storozhenko S, Steene JVD, Straeten DVD, Lambert WE. '''Free and total para-aminobenzoic acid analysis in plants with high-performance liquid chromatography/tandem mass spectrometry.''' Rapid Communications in Mass Spectrometry: Volume 19, Issue 8 , Pages 963 - 969, 2005. | |
| - | + | ||
| - | + | [9] Sybesma W, Burgess C, Starrenburg M, van Sinderen D, and Hugenholtz J. '''Multivitamin production in Lactococcus lactis using metabolic engineering.''' Metab Eng 2004 Apr; 6(2) 109-15. | |
| + | }} | ||
Latest revision as of 18:48, 20 October 2008
|
People
|
In vivo Folate Production
Background Information on FolateFolate, the generic term for the various forms of Vitamin B9, is an essential vitamin involved in single-carbon transfer reactions, which are important for many pathways including amino acid synthesis. Folate deficiencies in women can result in birth defects such as neural tube defects and other spinal cord malformations. As important as folate is, humans are unable to produce folate, and so must obtain it from eating foods such as green leafy vegetables or folate-fortified cereals. An engineered strain of bacteria that would constantly release folate into the gut would reduce the need to fortify breads and cereals with folate, as well as reduce folate-related birth defects in regions with little access to folate-containing foods. In addition to all the reasons stated above, folate is an ideal vitamin to be produced in the gut because, unlike many other vitamins, it has been shown to be absorbed in physiologically relevant quantities in the large intestine1, 2. Structurally, folate consists of three main parts: pteridine (derived from GTP), p-aminobenzoic acid (PABA, derived from chorismate), and a poly-glutamyl tail (derived from linking glutamate). Folate Biosynthesis PathwayAlthough folate is naturally produced in E. coli, the folate biosynthesis pathway in the bacteria Lactococcus lactis has been more heavily characterized and studied. In previous studies, this folate gene cluster has been successfully transformed into the folate-consuming bacteria L. gasseri, turning the bacteria into folate-producers4. Using the folate gene cluster from L. lactis also offers the additional benefit of removing the operon from its natural regulatory context. There are six major enzymatic activities involved in folate synthesis, which, in L. lactis, are contained in five genes: folB, folKE, folP, folC, and folA1. The first four, which we have chosen to focus on, are located in a gene cluster approximately 4.4kb long. We have chosen not to test overexpression of folA because it encodes for an enzyme which turns one form of folate (dihydrofolate) into another form of folate (tetrahydrofolate). Since our assay would detect both types of folate as part of the total folate production, folA was not a prime target for overexpression of folate. Our strategy is to clone the entire folate operon from the L.lactis genome and to transform the entire operon into E.coli. However, because we are unsure of whether or not the ribosomal binding sites (RBS) within the L.lactis operon would work in E.coli, we are also going to unpack the operon by cloning each of the four genes individually, placing them behind E.coli RBSs, and then recombine them into a single empty BioBricks™ plasmid. In addition to the main folate operon, we will also be experimenting with overexpression of the para-aminobenzoic acid (pABA) synthesis pathway from chorismate. Wegkamp et al. have shown that in order to increase overall total levels of folate, both the pABA synthesis genes and certain folate production genes need to be overexpressed5. The pABA pathway involves three enzymatic activities, pabA, pabB, and pabC – though in L.lactis, pabB is actually a bifunctional enzyme containing the activities for both pabB and pabC5. System DesignThe overall system design for testing folate production in E. coli is to construct several plasmids: one for each individual gene, one for the folate biosynthesis pathway, and one for the PABA synthesis pathway. Each gene, or gene cluster, would be cloned in an inducible-copy plasmid, which would be low copy by default, but can be switched to high copy via the addition of Isopropyl β-D-1-thiogalactopyranoside (IPTG)to the media. This will allow us to test overexpression of each plasmid separately. In addition, each plasmid contains a constitutive promoter, to ensure the constant production of folate. Folate Detection MethodsWe measured folate production, and thus the relative success of our engineering efforts, via a microbiological assay involving the folate-dependent strain Lactobacillus rhamnosus (ATCC 7469)6. The assay uses growth of the folate-dependent strain L. rhamnosus as an indicator of folate concentrations in the sample. The assay media specifically lacks folate, so folate becomes the limiting factor in the growth of L. rhamnosus, with the only possible source being the bacterial lysates of the engineered E. coli. In order to quantify the relative growth of the folate-dependent strain L. rhamnosus, a standard growth curve must first be characterized using known quantities of folic acid in assay media. Once the standard curve has been established, then experimental growth levels, as quantified by spectrophotometry, can be interpolated. Protocols are based off of BD Biosciences Folate Assay Medium datasheet7. Growth was measured by taking the OD at 546nm. Further details are available on the folate microbiological assay protocol page. pABA Detection Methodspara-Aminobenzoic Acid (PABA) can be detected using high performance liquid chromatography (HPLC)8. Using a 14 minute protocol, we were able to detect PABA peaks coming off a C18 column at 4.9 min. A standard curve was made by running a series of 1:3 PABA dilutions starting at a 10μg/ml concentration. The PABA for the standard curve was spiked into wild type E. coli cell lysate, which by itself did not show any detectable PABA peaks. Samples were run using the same HPLC protocol as the standards, and both lysate and supernatant were tested for PABA peaks. Again, details on the PABA detection method can be found on the para-aminobenzoic acid (pABA) HPLC protocol page. ResultsModifications to System DesignWe were able to successfully extract and clone the following three genes: folB, folKE, and pabA. We aimed to create individual constructs of each gene in the IPTG inducible-copy plasmid pSB2K3, as well as constructs with the two folate genes combined and with the two PABA synthesis genes combined. However, we had issues cloning our genes into the pSB2K3 + B0015 terminator construct, and so switched to completing the constructs in the high copy plasmid pSB1AK3 + B0015 terminator. Our final constructs, shown on the right, are folB in pSB2K3, and folKE and pabA in pSB1AK3. We were unable to sequence confirm our folBKE and pabB constructs, and so unfortunately could not include their data. Previous studies on folate overexpression have shown that both folate synthesis and PABA synthesis genes need to be overexpressed simultaneously in order to increase total folate levels3. Therefore, tests on folB and folKE were done with and without the addition of PABA during the E. coli inoculation; detection of higher folate levels on addition of PABA would indicate PABA as a limiting factor for folate production. We were unable to use PCR to extract several of the target genes: the entire folate gene cluster, folP, and folC. After analyzing the sequencing results of our other folate biosynthesis genes, we realized that the genetic sequences had several point mutations which were not `stop' codons. This indicated that we had a homologous, but not identical, genomic DNA than the one that we has used for PCR primer design. We believe that this homologous sequence could have resulted in too many differences between the primer and the genomic DNA, resulting in little to no binding. This may explain why we were unable to successfully extract folP, folC, and subsequently, the entire folate gene cluster (since folP and folC are the last two genes in the cluster) from the genomic DNA of L. lactis. The revised target constructs are shown .
Folate Assay Results for folB AND folKEThe experimental setup included running a standard curve with known amounts of folic acid (0-10ng) simultaneously with the samples, in order to have the same basis for comparison. The hope was that the standard curve growth OD values would correspond with known concentrations, and so sample concentrations could be interpolated based upon the standard. folKE, which was cloned into a constitutive high-copy plasmid, was tested with and without the addition of 500 ng of PABA during inoculation. folB, which is in an inducible copy plasmid, it was tested induced to high-copy as well as uninduced low-copy, with and without 500 ng of PABA during inoculation. We assayed both the supernatant and the cell lysate, though only the supernatant had measurable results. The growth data for the samples are very encouraging since the relative folate levels match what we would expect. We see that the addition of 500 ng of PABA during inoculation dramatically increases overall folate levels for folKE relative to the samples without PABA. Furthermore, adding PABA to the wild type controls did not affect growth at all, suggesting that the assay bacteria L.rhamnosus was not metabolizing the extra PABA. The extremely high OD values for all the samples were possibly the result of not completely washing out all of the culture media prior to adding the L.rhamnosus to the assay samples. When we repeated the assay in duplicate, this time on folB as well, we were able to observe the same trends in growth with and without the addition of PABA. Again, we see that for folKE, the addition of PABA does increase total folate levels, though not as dramatically as before. The rampant growth of the wild type control is disconcerting, but wild type folate production again appears to be unaffected by the addition of PABA. Furthermore, folate levels for folKE are still higher than the control where L.rhamnosus was added to only media without supernatant.
And what of folB? Recall that folB was the only gene to be successfully cloned into an inducible-copy plasmid, and so it was tested both induced and uninduced. The folate levels in the induced sample (high-copy) are higher than in the uninduced (low-copy) sample, which is consistent with what we would expect. The addition of PABA to both induced and uninduced increases relative levels of folate, which is also consistent. However, it is interesting to note that folate levels for the +PABA samples are the same for induced and uninduced. Of course, given the small sample size, this could just be due to variability, but it could also suggest that folate production reached an upper limit. This explanation seems even more likely if we reconsider the folate biosynthesis pathway, and we see that folB and folKE are both located upstream of actual integration of PABA into the molecule, accomplished by folP. It is very possible that we are increasing the flux of both pathways going into the folP junction, but as we were unable to overexpress folP as well, we may have created a bottleneck. para-Aminobenzoic Acid (pABA) HPLC Assay Results for pabAUsing high performance liquid chromatography (HPLC) we were able to detect PABA peaks from overexpression of pabA compared to a control sample of wild type supernatant. Compared to the peak areas of the standard curve, we were able to use a linear approximation to determine the concentration of PABA to be 703 ng/mL for pabA overexpression. Conclusions and Future WorkWe have shown, through very preliminary tests, the feasibility of overproduction of folate in E. coli using genes originally derived from L. lactis. Our data have confirmed previous studies in the necessity of overexpressing both folate and PABA synthesis genes, and we have shown that folate appears to be mostly present in the supernatant. Our data, again confirming previous results9, also suggest that overexpression of folKE is the most effective, definitely more effective than folB. We have also shown that it is possible to overexpress para-aminobenzoic acid production in E. coli and that overexpression of pabA increases total levels of para-aminobenzoic acid (PABA). Further work on this project would include repeating the folate assay to generate more data, perfecting the assay in order to quantify folate levels with the standard curve, making the pabA + pabB and folB + folKE constructs, making a folB + folKE + pabA + pabB construct, and extracting and cloning folP from the L. lactis genome. Relevant PartsBasic Parts
Construction Intermediates
Composite Parts
References[1] Asrar FM and O’Connor DL. Bacterially synthesized folate and supplemental folic acid are absorbed across the large intestine of piglets. J Nutr Biochem 2005 Oct; 16(10) 587-93. [2] Camilo E, Zimmerman J, Mason JB, Golner B, Russell R, Selhub J, and Rosenberg IH. Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology 1996 Apr; 110(4) 991-8. [3] Sybesma W, Starrenburg M, Kleerebezem M, Mierau I, de Vos WM, and Hugenholtz J. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl Environ Microbiol 2003 Jun; 69(6) 3069-76. [4] Wegkamp A, Starrenburg M, de Vos WM, Hugenholtz J, and Sybesma W. Transformation of folate-consuming Lactobacillus gasseri into a folate producer. Appl Environ Microbiol 2004 May;70(5) 3146-8. [5] Wegkamp A, van Oorschot W, de Vos WM, and Smid EJ. Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis. Appl Environ Microbiol 2007 Apr; 73(8) 2673-81. [6] Horne DW and Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem 1988 Nov; 34(11) 2357-9. [7] BD Biosciences. Folic Acid Assay Medium. [http://www.bd.com/ds/technicalCenter/inserts/Folic_Acid_Assay_Medium.pdf] [8] Zhang GF, Mortier KA, Storozhenko S, Steene JVD, Straeten DVD, Lambert WE. Free and total para-aminobenzoic acid analysis in plants with high-performance liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry: Volume 19, Issue 8 , Pages 963 - 969, 2005. [9] Sybesma W, Burgess C, Starrenburg M, van Sinderen D, and Hugenholtz J. Multivitamin production in Lactococcus lactis using metabolic engineering. Metab Eng 2004 Apr; 6(2) 109-15. |
 "
"