Transforming into Bacillus subtillis
From 2008.igem.org
(Difference between revisions)
Riachalder (Talk | contribs) |
|||
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | '' | + | {{:Team:Newcastle University/Header}} |
| + | {{:Team:Newcastle University/Template:UnderTheProtocol|page-title=[[Transforming into Bacillus subtillis|Transforming into ''Bacillus subtilis'']]}} | ||
| - | * Prepare two conical shake flasks of the following medium | + | |
| + | '''''B. subtilis'' cells require preparation to induce their natural competence to take up exogenous DNA. Begin the procedure the day before the transformation is planned.''' | ||
| + | |||
| + | * Prepare two conical shake flasks of the following medium: | ||
- 10mL SMM | - 10mL SMM | ||
| Line 16: | Line 20: | ||
* To one flask add 2mL liquid culture cells. | * To one flask add 2mL liquid culture cells. | ||
| - | * Incubate both flasks at 37°C overnight. (It is important that the flask without cells in is kept at 37°C also as this is the dilution medium and must be pre-warmed at the same temperature as the culture to avoid temperature stress of the cells.) | + | * Incubate both flasks at 37°C overnight. (''It is important that the flask without cells in is kept at 37°C also as this is the dilution medium and must be pre-warmed at the same temperature as the culture to avoid temperature stress of the cells.'') |
| - | * Add 500 µl overnight culture to the pre-warmed dilution medium and leave to incubate at 37 °C for 3 hours. (This allows the cells to enter the stationary growth phase.) | + | * Add 500 µl overnight culture to the pre-warmed dilution medium and leave to incubate at 37 °C for 3 hours. (''This allows the cells to enter the stationary growth phase.'') |
| - | * Add 10mL SMM, 125 µl sol E and 60 µl sol F to the dilute culture and leave to incubate at 37°C for a further 2 hours. (This starves the cells of amino acids such as tryptophan and CAA.) | + | * Add 10mL SMM, 125 µl sol E and 60 µl sol F to the dilute culture and leave to incubate at 37°C for a further 2 hours. (''This starves the cells of amino acids such as tryptophan and CAA.'') |
* Transfer 10µl plasmid to be transformed into a 2mL tube. Add 400µl nutrient-starved cell culture and mix by inverting the tube five times. | * Transfer 10µl plasmid to be transformed into a 2mL tube. Add 400µl nutrient-starved cell culture and mix by inverting the tube five times. | ||
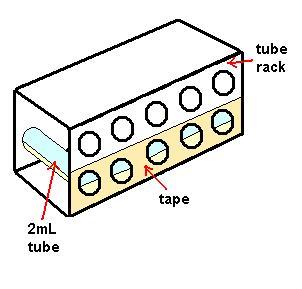
| - | * Incubate at 37°C whilst shaking for 1 hour. (The tubes must be laid on their side to properly aerate the medium. To do this, tape the tubes to the rack and lay it on its side to prevent the tubes falling out whilst shaking (see diagram).) | + | * Incubate at 37°C whilst shaking for 1 hour. (''The tubes must be laid on their side to properly aerate the medium. To do this, tape the tubes to the rack and lay it on its side to prevent the tubes falling out whilst shaking (see diagram).'') |
[[Image:amazing tube rack.jpg]] | [[Image:amazing tube rack.jpg]] | ||
| Line 27: | Line 31: | ||
* Pipette remaining liquid and pellet up and down to resuspend. | * Pipette remaining liquid and pellet up and down to resuspend. | ||
* Plate onto agar. | * Plate onto agar. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Latest revision as of 16:09, 28 October 2008
Newcastle University
GOLD MEDAL WINNER 2008
| Home | Team | Original Aims | Software | Modelling | Proof of Concept Brick | Wet Lab | Conclusions |
|---|
Home >> Wet Lab >> Protocols >> Transforming into Bacillus subtilis
B. subtilis cells require preparation to induce their natural competence to take up exogenous DNA. Begin the procedure the day before the transformation is planned.
- Prepare two conical shake flasks of the following medium:
- 10mL SMM
- 125 µl sol E (40% glucose)
- 100 µl tryptophan
- 60 µl sol F
- 10 µl casamino acid (CAA)
- 5µl ammonium iron
- To one flask add 2mL liquid culture cells.
- Incubate both flasks at 37°C overnight. (It is important that the flask without cells in is kept at 37°C also as this is the dilution medium and must be pre-warmed at the same temperature as the culture to avoid temperature stress of the cells.)
- Add 500 µl overnight culture to the pre-warmed dilution medium and leave to incubate at 37 °C for 3 hours. (This allows the cells to enter the stationary growth phase.)
- Add 10mL SMM, 125 µl sol E and 60 µl sol F to the dilute culture and leave to incubate at 37°C for a further 2 hours. (This starves the cells of amino acids such as tryptophan and CAA.)
- Transfer 10µl plasmid to be transformed into a 2mL tube. Add 400µl nutrient-starved cell culture and mix by inverting the tube five times.
- Incubate at 37°C whilst shaking for 1 hour. (The tubes must be laid on their side to properly aerate the medium. To do this, tape the tubes to the rack and lay it on its side to prevent the tubes falling out whilst shaking (see diagram).)
- Centrifuge tube at 13,000g for 2 minutes to pellet the cells.
- Remove and discard 300µl supernatent. Re-centrifuge if pellet has already resuspended.
- Pipette remaining liquid and pellet up and down to resuspend.
- Plate onto agar.
 "
"