Team:Imperial College/Cloning Strategy
From 2008.igem.org
m |
|||
| (5 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Imperial/StartPage2}} | {{Imperial/StartPage2}} | ||
| - | + | === Cloning Strategy === | |
| - | {{Imperial/Box2| | + | {{Imperial/Box2|| |
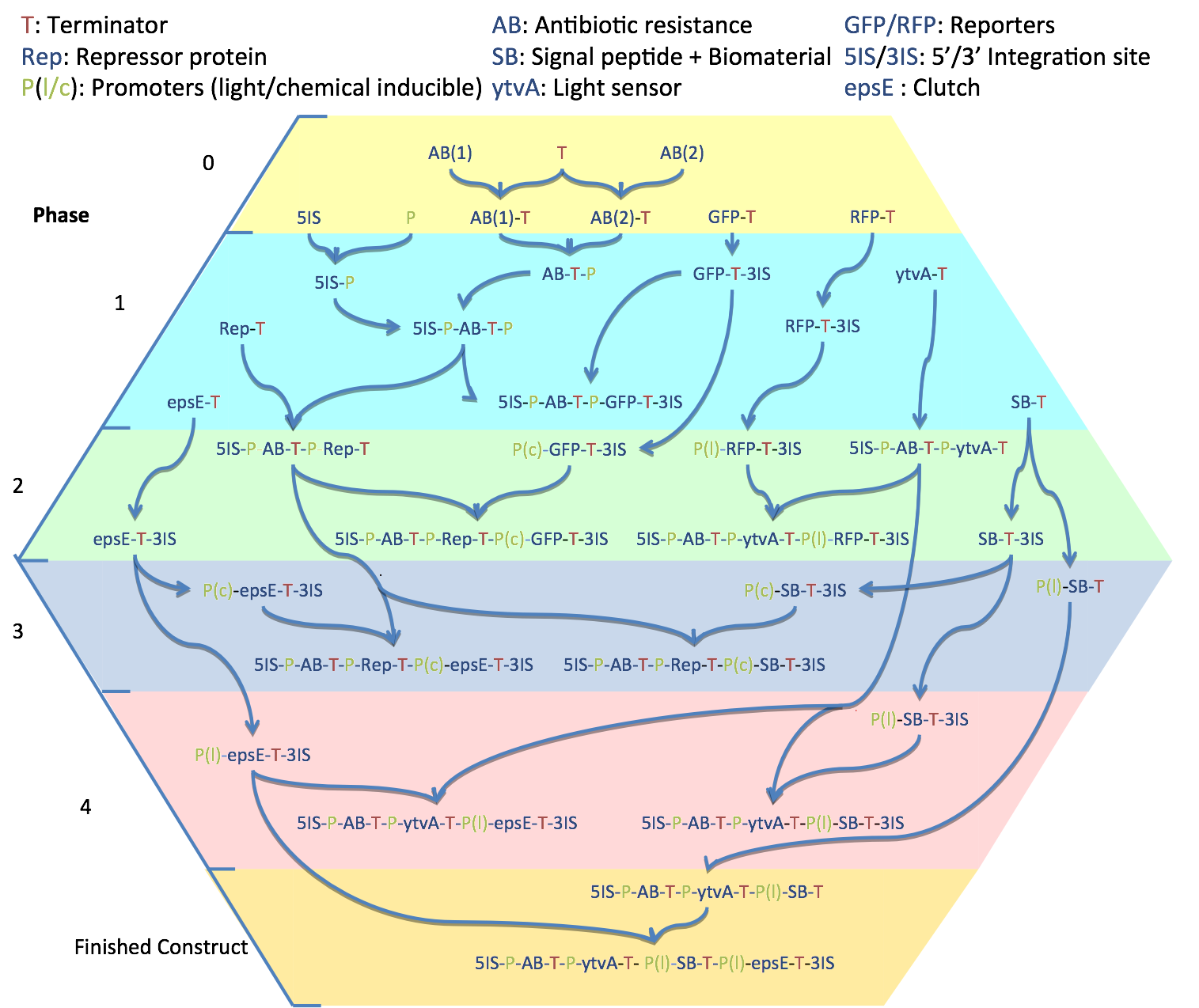
The Imperial iGEM 2008 team faces the task of working with a chassis that has been rarely used - and never characterised - in the competition to date. While the ''B. subtilis'' chassis offers us many advantages, working from the ground up also presents many challenges.}} | The Imperial iGEM 2008 team faces the task of working with a chassis that has been rarely used - and never characterised - in the competition to date. While the ''B. subtilis'' chassis offers us many advantages, working from the ground up also presents many challenges.}} | ||
| Line 17: | Line 17: | ||
We will test 4 combinations of 2 constitutive promoters and 2 RBSs to characterise them. An antibiotic resistance cassette is placed at the 5' end of the construct, to prevent any readthrough by the native trancriptases from reaching the regulated BioBricks. | We will test 4 combinations of 2 constitutive promoters and 2 RBSs to characterise them. An antibiotic resistance cassette is placed at the 5' end of the construct, to prevent any readthrough by the native trancriptases from reaching the regulated BioBricks. | ||
| - | <html><img width=" | + | <html><center><img width="750px" src="http://i59.photobucket.com/albums/g305/Timpski/Phase1.png"></center></html> |
====== Phase 2: Testing of Inducible Promoters ====== | ====== Phase 2: Testing of Inducible Promoters ====== | ||
Promoters marked with a 'C' are chemically-inducible and those marked with an 'L' are light-inducible. Since YtvA responds to blue light, fluorescence from GFP may cause positive feedback. We will therefore be using RFP as a quantifiable output to test our light-inducible promoters instead. 'Rep' genes encode a repressor for the chemically-inducible promoters to stop leaky expression. | Promoters marked with a 'C' are chemically-inducible and those marked with an 'L' are light-inducible. Since YtvA responds to blue light, fluorescence from GFP may cause positive feedback. We will therefore be using RFP as a quantifiable output to test our light-inducible promoters instead. 'Rep' genes encode a repressor for the chemically-inducible promoters to stop leaky expression. | ||
| - | <html><img width=" | + | <html><center><img width="750px" src="http://i59.photobucket.com/albums/g305/Timpski/Phase2A.png"> |
| - | <img width=" | + | <img width="750px" src="http://i59.photobucket.com/albums/g305/Timpski/Phase2B.png"></center></html> |
====== Phase 3: Clutch and Biomaterial Characterisation ====== | ====== Phase 3: Clutch and Biomaterial Characterisation ====== | ||
Testing and characterisation of the clutch (EpsE, produced by the ''epsE'' gene) and biomaterial synthesis (SB - signal peptide and biomaterial) using a chemically-inducible promoter. The promoter is otherwise constitutively repressed by Rep. | Testing and characterisation of the clutch (EpsE, produced by the ''epsE'' gene) and biomaterial synthesis (SB - signal peptide and biomaterial) using a chemically-inducible promoter. The promoter is otherwise constitutively repressed by Rep. | ||
| - | <html><img width=" | + | <html><center><img width="750px" src="http://i59.photobucket.com/albums/g305/Timpski/Phase3A.png"> |
| - | <img width=" | + | <img width="750px" src="http://i59.photobucket.com/albums/g305/Timpski/Phase3B.png"></center></html> |
====== Phase 4: Device Characterisation ====== | ====== Phase 4: Device Characterisation ====== | ||
Combination of light induction and EpsE/biomaterial expression. The clutch (EpsE) and biomaterial are now under control of the light-inducible activator sigma B (via YtvA). | Combination of light induction and EpsE/biomaterial expression. The clutch (EpsE) and biomaterial are now under control of the light-inducible activator sigma B (via YtvA). | ||
| - | <html><img width=" | + | <html><center><img width="750px" src="http://i59.photobucket.com/albums/g305/Timpski/Phase4A.png"> |
| - | <img width=" | + | <img width="750px" src="http://i59.photobucket.com/albums/g305/Timpski/Phase4B.png"></center></html> |
====== Final Construct: System Characterisation ====== | ====== Final Construct: System Characterisation ====== | ||
Combination of light sensing and light-induced expression of epsE and biomaterial. Each gene has its own promoter/RBS pair because in ''B. subtilis'', it has been shown that levels of expression decrease as one moves along an operon. | Combination of light sensing and light-induced expression of epsE and biomaterial. Each gene has its own promoter/RBS pair because in ''B. subtilis'', it has been shown that levels of expression decrease as one moves along an operon. | ||
| - | <p><html><img width=" | + | <p><html><center><img width="750px" src="http://i59.photobucket.com/albums/g305/Timpski/S1L.png"></center></html></p> |
}} | }} | ||
{{Imperial/EndPage|Wet_Lab|Protocols}} | {{Imperial/EndPage|Wet_Lab|Protocols}} | ||
Latest revision as of 11:11, 22 April 2009
Cloning Strategy
|
|||||||||||||||
 "
"