September
From 2008.igem.org
WeberSimone (Talk | contribs) |
m |
||
| (3 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
Content= | Content= | ||
<div style="font-size:18pt;"> | <div style="font-size:18pt;"> | ||
| - | <font face="Arial Rounded MT Bold" style="color:#010369"> | + | <font face="Arial Rounded MT Bold" style="color:#010369">_september</font></div> |
<br> | <br> | ||
<br> | <br> | ||
| Line 32: | Line 32: | ||
<h3>Sep. 11th 2008</h3> | <h3>Sep. 11th 2008</h3> | ||
| - | [[image:11.09.08 Test Fura staining-Freigem08.jpg | | + | [[image:11.09.08 Test Fura staining-Freigem08.jpg |600px]]<br> |
<br> | <br> | ||
| - | [[image:11.09.08 Test Bindung Origami to alexa-Freigem08.jpg | | + | [[image:11.09.08 Test Bindung Origami to alexa-Freigem08.jpg |600px]]<br> |
| Line 130: | Line 130: | ||
'''6) CMV PCR''' (Sabine)<br> | '''6) CMV PCR''' (Sabine)<br> | ||
-the same PCR with different annealing temperatures using a gradient between 58°C and 62°C (optimal annealing temperature: 62°C)<br> | -the same PCR with different annealing temperatures using a gradient between 58°C and 62°C (optimal annealing temperature: 62°C)<br> | ||
| - | -again, no products were gained | + | -again, no products were gained<br><br> |
| + | |||
| + | '''7) preparation of the 293T cells for a Mg2+ and TAE-tolerancetest (MTT-assay)''' Normann | ||
| + | <ul> <li> scraping cells off the dish and centrifuging them at 1300rpm (5min) | ||
| + | <li> discarding the supernatant and washing the cells in 10ml PBS | ||
| + | <li> spinning down again at 1300rpm (5min), discarding the supernatant and solving the cells in 10ml fresh medium (DMEM) | ||
| + | <li> giving 500µl of the cellsuspension to each well of a 6 well plate and adding Medium and 125mM MgAc | ||
| + | </ul> | ||
| + | <table style="text-align: left; width: 100px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>Well</td> | ||
| + | <td>Medium added [µl]</td> | ||
| + | <td>MgAc (125mM) added [µl]</td> | ||
| + | <td>final conentration of Mg2+[mM]</td> | ||
| + | <td>TA added ( for 30min at 16.9.08)</td> | ||
| + | |||
| + | </tr> | ||
| + | <tr><td>1</td> | ||
| + | <td>4500</td> <td>0</td> | ||
| + | <td>0</td> | ||
| + | <td>No</td> | ||
| + | </tr> | ||
| + | <tr><td>2</td> <td>4500</td> <td>0</td> | ||
| + | <td>0</td> | ||
| + | <td>Yes</td> | ||
| + | </tr> | ||
| + | <tr><td>3</td> <td>4000</td> <td>500</td> | ||
| + | <td>12,5</td> | ||
| + | <td>Yes</td> | ||
| + | </tr> | ||
| + | <tr><td>4</td> <td>4250</td> <td>250</td> | ||
| + | <td>6,25</td> | ||
| + | <td>Yes</td> | ||
| + | </tr> | ||
| + | <tr><td>5</td> <td>4250</td> <td>250</td> | ||
| + | <td>6,25</td> | ||
| + | <td>No</td> | ||
| + | </tr> | ||
| + | <tr><td>6</td> <td>4000</td> <td>500</td> | ||
| + | <td>12,5</td> | ||
| + | <td>No</td> | ||
| + | </tr> | ||
| + | </table> | ||
| Line 227: | Line 270: | ||
-Venus split C-GFP<br> | -Venus split C-GFP<br> | ||
-Cerulan split N-CFP<br> | -Cerulan split N-CFP<br> | ||
| + | |||
| + | <h3>Sep. 30th 2008</h3><br> | ||
| + | '''Maesurement of calcium influx<br>'''(Simone) | ||
| + | Cell staining<br> | ||
| + | 1)wash cells in medium (RPMI) with 1% FCS<br> | ||
| + | 2)count the cells and then resuspend the cells in RPMI with 1%FCS -> concentration 10^6 – 5x 10^6 cells/ml!<br> | ||
| + | 3)Mix:<br> | ||
| + | 25µl Indo-1 (5µg/ml)<br> | ||
| + | 25µl Pluronic F127 (0,5µg/ml)<br> | ||
| + | 113µl FCS<br> | ||
| + | -> mix it very well and incubate it for 5minutes<br> | ||
| + | 4)Add 15µl of the mixture/ml of cells -> incubate it in the dark for 45minutes at 37°C, shake it every 15minutes<br> | ||
| + | 5)Add 5-10ml medium (use the same medium as the one which will be used for the measurement) and centrifuge it at 1200rpm for 5minutes<br> | ||
| + | 6)Take supernatant out. And resuspend the cells to 2-5x10^6 cells/ml<br> | ||
| + | <br> | ||
| + | Measurement on the FACS<br> | ||
| + | Ca2+ response was induced by addition of the indicated stimulus 1 min after starting to record the ratio of Ca2+-bound Indo-1 versus unbound Indo-1 with a LSRII fluorescence spectrometer (Becton Dickinson). Data were analyzed with the FloJo 6.1 software.<br> | ||
| + | For the measurement: prewarm 50ml of the medium for the measurement up to 37°C, to make sure that you have the optimal conditions for the cells.<br> | ||
| + | <br> | ||
| + | Results<br> | ||
| + | [[Image:Image-Freiburg2008_FACS_result.jpg|600px]] | ||
}} | }} | ||
Latest revision as of 02:41, 30 October 2008
|
_september
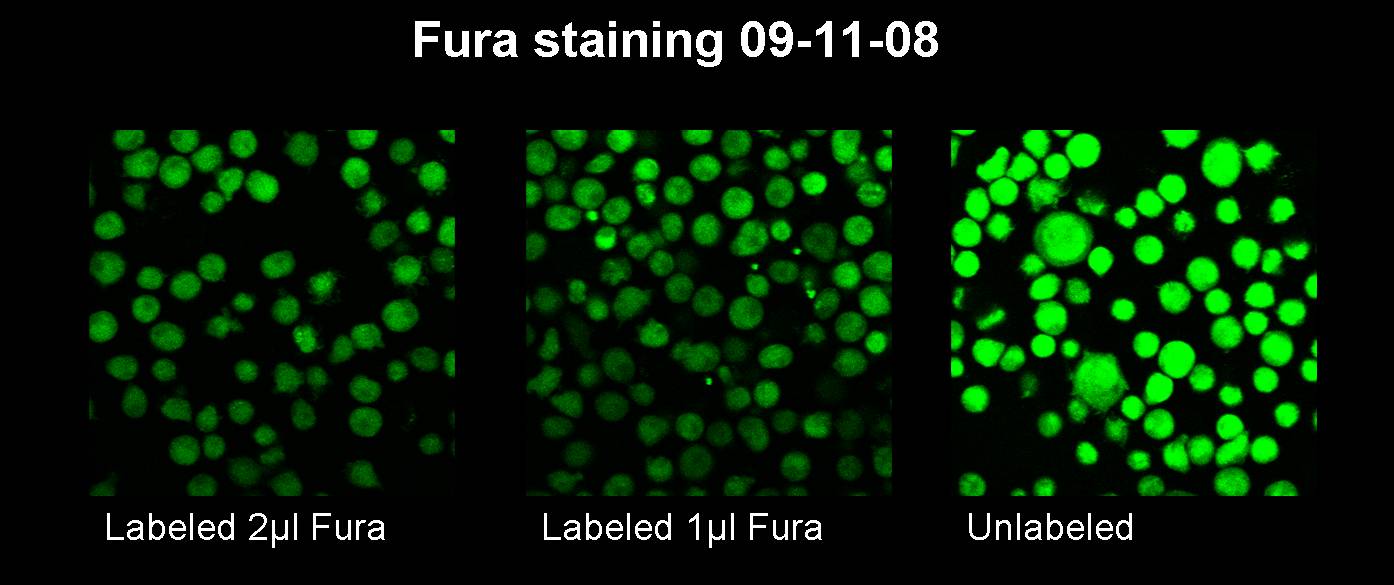
Sep. 10th 2008
For a 50 µl reaction: The settings for the PCR machine are the following: No product was received. Sep. 11th 2008
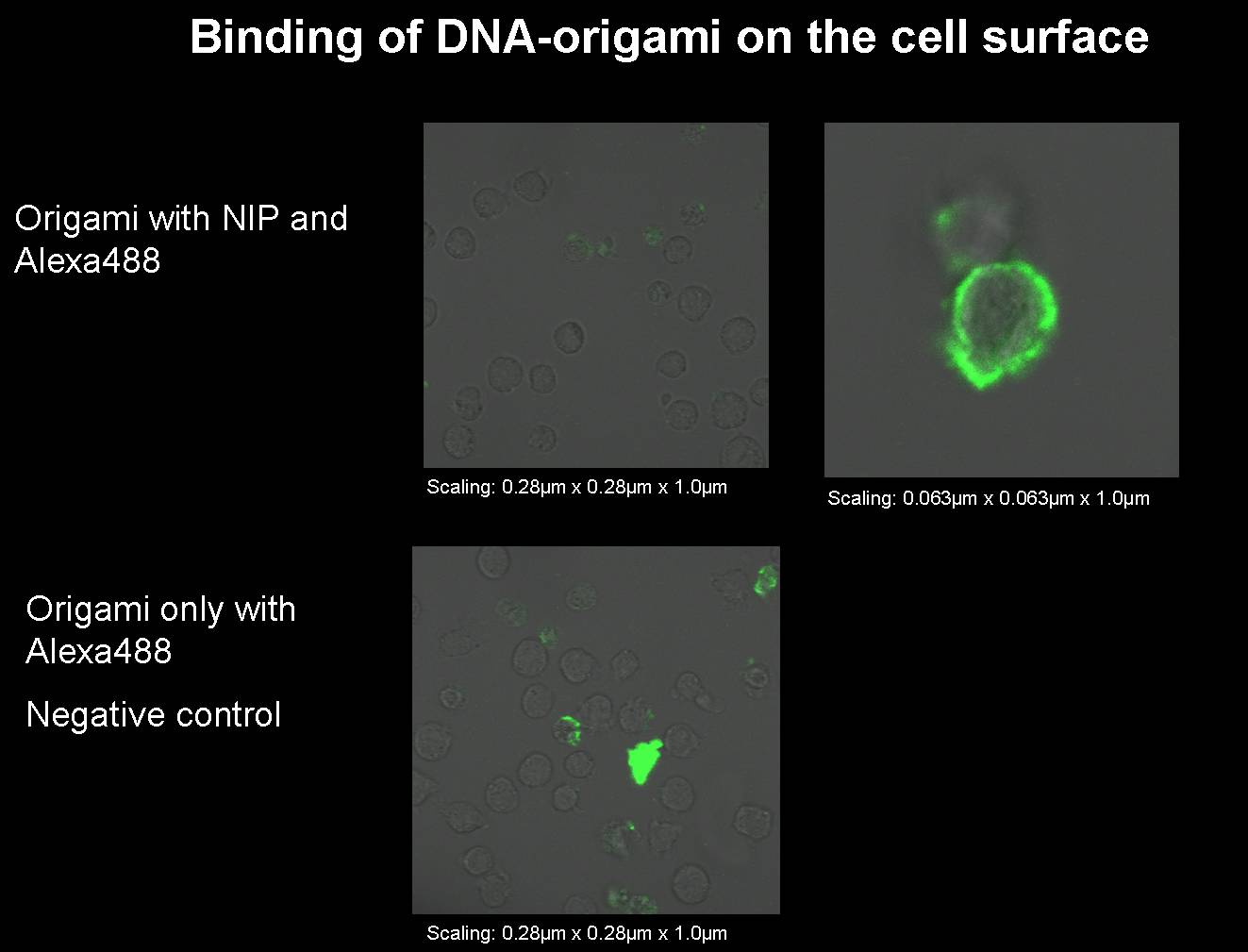
Sep. 12th 20081) Origami with NIP and fluorophor for the binding measurement (Norman+Simone) We had to produce some new origami for our next binding measurements.
see at the protocol from 07-24-2008 2) Origami for the Calciummeasurement (Norman+Simone)
see at the protocol from 07-24-2008 To increase the concentration of origami we also made to probes with the double amount ingredients of the protocol from 07.24.2008
3) Master cycler (Norman+Simone) The origamis were produced in the mastercycler as explained before. 4) Purification of the DNA Origami (Norman+Simone) Was done as before 5) Digestion of CMV+Rluc (Sabine) Digestion with EcoRV und FspI (3h at 37°C)
File:Verdau CMVRluc EcoRV FspI klein.jpg
7) preparation of the 293T cells for a Mg2+ and TAE-tolerancetest (MTT-assay) Normann
Sep. 15th 2008CMV-PCR (Sabine)
Sep. 16th 2008Gel purification (Sabine) Digestion of the PCR products and the transfectionvector (Sabine, Kathrin)
Sep. 18th 2008Transformation (Sabine)
Sep. 19th 2008Transformation (Sabine)
Sep. 20th 2008Digestion of the PCR products and the transfection-vector (Sabine) Gel purification and ligation (Sabine)
Sep. 21st 2008Transformation of the ligation (Sabine) Sep. 23rd 20081. Transformation of the 4 plasmids of ATG (michael) Sep. 24th 20081. Transformation (michael) Sep. 25th 2008Miniprep (normann) Sep. 26th 2008Transformation of the 'ligation 2nd try' (michael) Sep. 30th 2008Maesurement of calcium influx |
 "
"