Team:NTU-Singapore/Parts/Standard Promoters
From 2008.igem.org
(→Transformation of parts) |
Lalala8585 (Talk | contribs) |
||
| (12 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
<div id="body" style="margin-top:200px;width:950px;padding:5px;"> | <div id="body" style="margin-top:200px;width:950px;padding:5px;"> | ||
| - | = | + | ='''Standard Promoters'''= |
| - | In the first edition of the newsletter, it elaborated a method similar to the approach our characterization procedure. We have decided to make use of the protocol given to us, however with some minor modifications. | + | In the first edition of the [http://partsregistry.org/Measurement/SPU/Measure iGEM newsletter], it elaborated a method that was similar to the approach that our team had adopted for our own characterization procedure. We have decided to make use of the protocol given to us, however with some minor modifications. |
| - | =Transformation of parts= | + | |
| + | [[Image:NTU@iGEM_MIT_300px-Promoter_Instructions_Measure.png|thumb|center|300px| iGEM Newsletter]]<br> | ||
| + | |||
| + | |||
| + | =='''Transformation of parts'''== | ||
The parts transformed are: | The parts transformed are: | ||
#pLacI-GFP [http://partsregistry.org/wiki/index.php?title=Part:BBa_J04430 BBa_J04430], | #pLacI-GFP [http://partsregistry.org/wiki/index.php?title=Part:BBa_J04430 BBa_J04430], | ||
| Line 14: | Line 18: | ||
#Medium promoter [http://partsregistry.org/Part:BBa_J23151 BBa_J23151] and | #Medium promoter [http://partsregistry.org/Part:BBa_J23151 BBa_J23151] and | ||
#Strong promoter [http://partsregistry.org/Part:BBa_J23102 BBa_J23102] | #Strong promoter [http://partsregistry.org/Part:BBa_J23102 BBa_J23102] | ||
| - | ===Discussion=== | + | ==='''Discussion'''=== |
With the parts transformed, we can proceed with the characterization based on the protocol we constructed. We make use of the FLx800™ Fluorescence Microplate Reader to take the readings of the fluorescence that is emitted by the GFP reporting devices by measuring the relative fluorescence units (RFU). The different strength of the promoters associated with the same GFP reporting device, E0240 will determine the rate of transcription of the GFP protein. The stronger the promoter, the higher is the rate of its transcription. This results in more GFP proteins formed which in turns relates to more fluorescence being emitted. This direct proportionality brings us closer to quantifying the strength of any promoter of interest. Hence a standard promoter was used to compare the RFU with other promoters. Based on the newsletter, there are also the Low, Medium and High promoters. | With the parts transformed, we can proceed with the characterization based on the protocol we constructed. We make use of the FLx800™ Fluorescence Microplate Reader to take the readings of the fluorescence that is emitted by the GFP reporting devices by measuring the relative fluorescence units (RFU). The different strength of the promoters associated with the same GFP reporting device, E0240 will determine the rate of transcription of the GFP protein. The stronger the promoter, the higher is the rate of its transcription. This results in more GFP proteins formed which in turns relates to more fluorescence being emitted. This direct proportionality brings us closer to quantifying the strength of any promoter of interest. Hence a standard promoter was used to compare the RFU with other promoters. Based on the newsletter, there are also the Low, Medium and High promoters. | ||
| - | ===Results=== | + | ==='''Results'''=== |
| - | + | Firstly, the parts in listed above were transformed into the Top 10 competent cells. | |
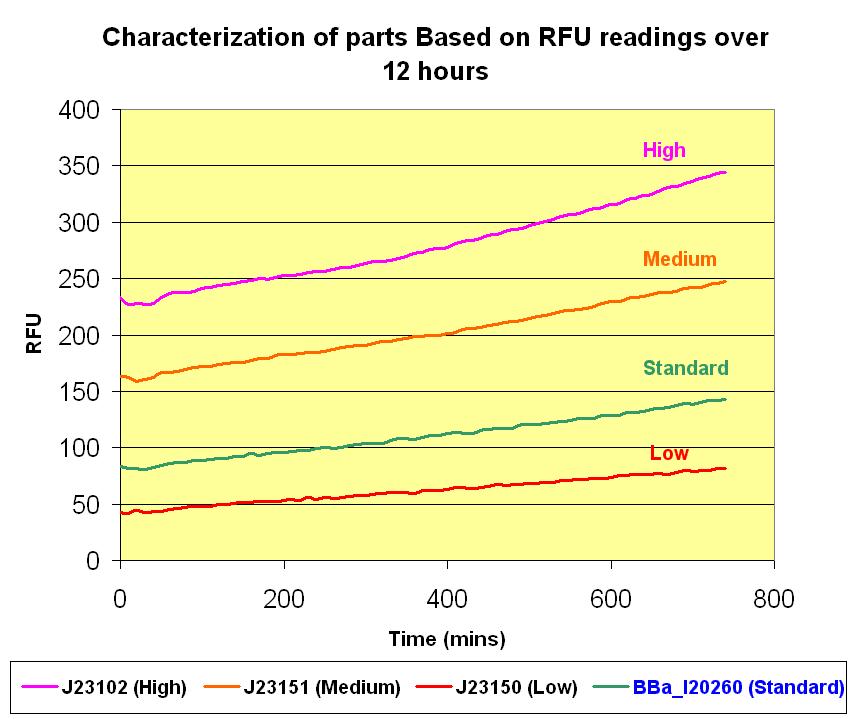
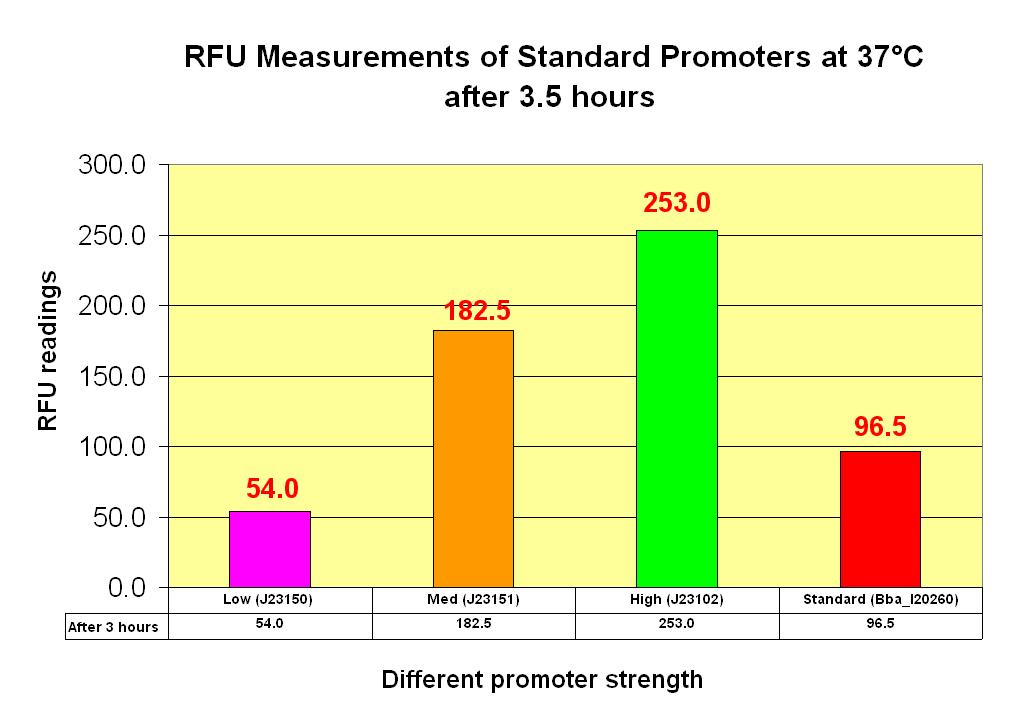
| + | After which, for a time duration of 12 hours, readings were taken at 10 minutes intervals. The graphs below illustrate the results we obtained and the detailed data points and graphs plotting can be retrieved from the [[image: NTU@iGEM_Results_for_characterization_pLacI_010708.xls]]. | ||
[[image: NTU_Characterization_Graph_Standards_(12_hours).JPG |thumb|center|600px|Characterization Graph of parts over 12 hours]] | [[image: NTU_Characterization_Graph_Standards_(12_hours).JPG |thumb|center|600px|Characterization Graph of parts over 12 hours]] | ||
| - | [[image: NTU_Characterization_Graph_Standards_(3_hours).JPG|thumb| | + | [[image: NTU_Characterization_Graph_Standards_(3_hours).JPG|thumb|left|450px|Characterization Graph of parts after 3 hours]] |
| - | [[image: NTU_Characterization_Graph_Standards_(3.5_hours).JPG|thumb| | + | [[image: NTU_Characterization_Graph_Standards_(3.5_hours).JPG|thumb|right|450px|Characterization Graph of parts after 3.5 hours]] |
| + | <br><br> | ||
| + | <html> | ||
| + | <script language=Javascript1.2> | ||
| + | <!-- | ||
| + | |||
| + | var tags_before_clock = "<b>It is now " | ||
| + | var tags_middle_clock = "on" | ||
| + | var tags_after_clock = "</b>" | ||
| + | |||
| + | if(navigator.appName == "Netscape") { | ||
| + | document.write('<layer id="clock"></layer><br>'); | ||
| + | } | ||
| + | |||
| + | if (navigator.appVersion.indexOf("MSIE") != -1){ | ||
| + | document.write('<span id="clock"></span>'); | ||
| + | } | ||
| + | |||
| + | DaysofWeek = new Array() | ||
| + | DaysofWeek[0]="Sunday" | ||
| + | DaysofWeek[1]="Monday" | ||
| + | DaysofWeek[2]="Tuesday" | ||
| + | DaysofWeek[3]="Wednesday" | ||
| + | DaysofWeek[4]="Thursday" | ||
| + | DaysofWeek[5]="Friday" | ||
| + | DaysofWeek[6]="Saturday" | ||
| + | |||
| + | Months = new Array() | ||
| + | Months[0]="January" | ||
| + | Months[1]="February" | ||
| + | Months[2]="March" | ||
| + | Months[3]="April" | ||
| + | Months[4]="May" | ||
| + | Months[5]="June" | ||
| + | Months[6]="July" | ||
| + | Months[7]="August" | ||
| + | Months[8]="September" | ||
| + | Months[9]="October" | ||
| + | Months[10]="November" | ||
| + | Months[11]="December" | ||
| + | |||
| + | function upclock(){ | ||
| + | var dte = new Date(); | ||
| + | var hrs = dte.getHours(); | ||
| + | var min = dte.getMinutes(); | ||
| + | var sec = dte.getSeconds(); | ||
| + | var day = DaysofWeek[dte.getDay()] | ||
| + | var date = dte.getDate() | ||
| + | var month = Months[dte.getMonth()] | ||
| + | var year = dte.getFullYear() | ||
| + | |||
| + | var col = ":"; | ||
| + | var spc = " "; | ||
| + | var com = ","; | ||
| + | var apm; | ||
| + | |||
| + | if (date == 1 || date == 21 || date == 31) | ||
| + | {ender = "<sup>st</sup>"} | ||
| + | else | ||
| + | if (date == 2 || date == 22) | ||
| + | {ender = "<sup>nd</sup>"} | ||
| + | else | ||
| + | if (date == 3 || date == 23) | ||
| + | {ender = "<sup>rd</sup>"} | ||
| + | |||
| + | else | ||
| + | {ender = "<sup>th</sup>"} | ||
| + | |||
| + | if (12 < hrs) { | ||
| + | apm="<font size='-1'>pm</font>"; | ||
| + | hrs-=12; | ||
| + | } | ||
| + | |||
| + | else { | ||
| + | apm="<font size='-1'>am</font>"; | ||
| + | } | ||
| + | |||
| + | if (hrs == 0) hrs=12; | ||
| + | if (hrs<=9) hrs="0"+hrs; | ||
| + | if (min<=9) min="0"+min; | ||
| + | if (sec<=9) sec="0"+sec; | ||
| + | |||
| + | if(navigator.appName == "Netscape") { | ||
| + | document.clock.document.write(tags_before_clock+hrs+col+min+col+sec+apm+spc+tags_middle_clock+spc+day+com+spc+date+ender+spc+month+com+spc+year+tags_after_clock); | ||
| + | document.clock.document.close(); | ||
| + | } | ||
| + | |||
| + | if (navigator.appVersion.indexOf("MSIE") != -1){ | ||
| + | clock.innerHTML = tags_before_clock+hrs+col+min+col+sec+apm+spc+tags_middle_clock+spc+day+com+spc+date+ender+spc+month+com+spc+year+tags_after_clock; | ||
| + | } | ||
| + | } | ||
| + | |||
| + | setInterval("upclock()",1000); | ||
| + | //--> | ||
| + | </script> | ||
| + | </html> | ||
Latest revision as of 05:48, 27 October 2008
|
Contents |
Standard Promoters
In the first edition of the [http://partsregistry.org/Measurement/SPU/Measure iGEM newsletter], it elaborated a method that was similar to the approach that our team had adopted for our own characterization procedure. We have decided to make use of the protocol given to us, however with some minor modifications.
Transformation of parts
The parts transformed are:
- pLacI-GFP [http://partsregistry.org/wiki/index.php?title=Part:BBa_J04430 BBa_J04430],
- Standard promoter with GFP reporter [http://partsregistry.org/Part:BBa_I20260 BBa_I20260]
- GFP reporter devices with Weak promoter[http://partsregistry.org/Part:BBa_J23150 BBa_J23150],
- Medium promoter [http://partsregistry.org/Part:BBa_J23151 BBa_J23151] and
- Strong promoter [http://partsregistry.org/Part:BBa_J23102 BBa_J23102]
Discussion
With the parts transformed, we can proceed with the characterization based on the protocol we constructed. We make use of the FLx800™ Fluorescence Microplate Reader to take the readings of the fluorescence that is emitted by the GFP reporting devices by measuring the relative fluorescence units (RFU). The different strength of the promoters associated with the same GFP reporting device, E0240 will determine the rate of transcription of the GFP protein. The stronger the promoter, the higher is the rate of its transcription. This results in more GFP proteins formed which in turns relates to more fluorescence being emitted. This direct proportionality brings us closer to quantifying the strength of any promoter of interest. Hence a standard promoter was used to compare the RFU with other promoters. Based on the newsletter, there are also the Low, Medium and High promoters.
Results
Firstly, the parts in listed above were transformed into the Top 10 competent cells. After which, for a time duration of 12 hours, readings were taken at 10 minutes intervals. The graphs below illustrate the results we obtained and the detailed data points and graphs plotting can be retrieved from the File:NTU@iGEM Results for characterization pLacI 010708.xls.
 "
"