Team:Imperial College/Light Inducible
From 2008.igem.org
(Difference between revisions)
| (2 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Imperial/StartPage2}} | {{Imperial/StartPage2}} | ||
| - | + | ||
| - | + | {{Imperial/Box1|Characterization of Light Inducible Expression| | |
| - | === | + | ======Aims====== |
| - | + | ||
| - | === | + | |
The aim of this protocol is to characterise the light induced expression of GFPmut3b under the control of the ''pctc'' and ''pgsbi'' promoters. In addition to expression of these constructs, we require overexpression of the YtvA receptor protein. The complexity of the characterisation of light inducible promoters means that we need to carry out several levels of testing: | The aim of this protocol is to characterise the light induced expression of GFPmut3b under the control of the ''pctc'' and ''pgsbi'' promoters. In addition to expression of these constructs, we require overexpression of the YtvA receptor protein. The complexity of the characterisation of light inducible promoters means that we need to carry out several levels of testing: | ||
| Line 15: | Line 13: | ||
* If we Cannot excite the light receptor with the flourometer then what should we use? Could try to filter white light out using filters or cellophane or alternatively we could excite using intense white light. | * If we Cannot excite the light receptor with the flourometer then what should we use? Could try to filter white light out using filters or cellophane or alternatively we could excite using intense white light. | ||
| - | ==1. Testing overexpression of YtvA receptor== | + | ======1. Testing overexpression of YtvA receptor====== |
| - | + | ======Equipment====== | |
| - | ===Equipment=== | + | |
*Spectrometer, | *Spectrometer, | ||
| - | + | ======Reagents and Materials====== | |
| - | ===Reagents and Materials=== | + | |
*2x5ml LB media in 50ml flasks, | *2x5ml LB media in 50ml flasks, | ||
*2x10ml LB media in 100ml flasks, | *2x10ml LB media in 100ml flasks, | ||
*pipettes, | *pipettes, | ||
*cuvettes, | *cuvettes, | ||
| - | + | ======Protocol====== | |
| - | ===Protocol=== | + | |
'''Day 1'''<br> | '''Day 1'''<br> | ||
| - | + | #Pick a colony from a plate of ''B. subtilis'' and inoculate 5ml of LB media and grow overnight at 37<sup>o</sup>C. | |
| - | + | #Pick a colony from a plate of ''B. subtilis'' transformed with construct (define when list is up) and inoculate 5ml of LB media and grow overnight at 37<sup>o</sup>C. | |
| - | + | #Both flasks should be totally covered in foil to ensure that they are grown in darkness. | |
'''Day 2'''<br> | '''Day 2'''<br> | ||
| - | + | #Measure the O.D.<sub>600</sub> of both the overnight cultures using LB media as a blank. With the O.D.<sub>600</sub> calculate the dilution to achieve an O.D.<sub>600</sub> of 0.5 in 10ml (does not matter too much what O.D.<sub>600</sub> we choose, key is to standardise them) using the following calculation: | |
| - | + | #*Volume of Overnight Culture (X) {{Equals}} (0.5/O.D.<sub>600</sub>)*10ml | |
| - | + | #*Volume of fresh Culture (Y) {{Equals}} 10-X (Overnight Culture) | |
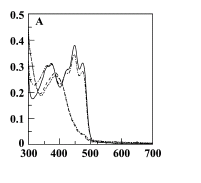
| - | + | #To determine whether the YtvA light receptor has been expressed correctly we can measure the absorption of the YtvA within each of the cultures. We should see an increase in absorption within the spectra of YtvA overexpressed culture (Figure 1). | |
| - | + | #The following wavelengths have been chosen across the absorption spectra of the YtvA: | |
| - | + | #*375nm (An Absorption Peak) | |
| - | + | #*450nm (Absorption Max) | |
| - | + | #*500nm (Not absorbed) | |
| - | + | #*550nm (Not absorbed) | |
| - | + | #To measure each of these, 1ml of the cultures should be pipetted into a cuvette and measured immediately in a spectrometer, with each wavelength being repeated twice. | |
| - | + | #What we should observe is an increase in absorption in the ''B. subtilis'' expressing YtvA in the absorption spectra and not in the wavelengths out of the spectra. | |
| + | [[Image:Spectra YtvA.PNG|200px|center|Figure 1. Spectra of YtvA]] | ||
| - | + | ======2.Testing the exposure time required to induce expression====== | |
| - | + | ======Equipment====== | |
| - | + | *Fluorometer | |
| - | + | ======Reagents and Materials====== | |
| - | ==2.Testing the exposure time required to induce expression== | + | |
| - | + | ||
| - | ===Equipment=== | + | |
| - | *Fluorometer | + | |
| - | + | ||
| - | ===Reagents and Materials=== | + | |
*2x10ml LB media in 100ml flasks | *2x10ml LB media in 100ml flasks | ||
*Pipettes | *Pipettes | ||
| Line 61: | Line 51: | ||
*96 well plate | *96 well plate | ||
*Plate lids | *Plate lids | ||
| - | + | ======Protocol====== | |
| - | ===Protocol=== | + | |
'''Day 1'''<br> | '''Day 1'''<br> | ||
| - | + | #Collect 10ml of LB media (containing suitable antibiotics) into a 100ml flask. Inoculate the media with a single colony from a ''B. subtilis'' plate and grow overnight at 37<sup>o</sup>.<br> | |
| - | + | #Grow this culture overnight in the dark by covering the flask in foil. | |
| - | + | ||
'''Day 2'''<br> | '''Day 2'''<br> | ||
| - | + | #Collect 1 x 100ml flasks containing 10ml of LB media (containing suitable antibiotics) and remove 1ml of the media and pipette into a blank cuvette (this is to make the blank for OD measurements). Remove 1ml of the overnight culture and measure the OD<sub>600</sub> | |
| - | + | #Mix thoroughly and remove 1ml of the culture and measure the OD<sub>600</sub>. Now we need to dilute the culture down to a suitable OD<sub>600</sub> of 0.2 in 10ml of culture, use the following calculation: | |
| - | + | #*Volume of Overnight Culture (X) {{Equals}} (0.5/O.D.<sub>600</sub>)*10ml | |
| - | + | #*Volume of fresh Culture (Y) {{Equals}} 10-X (Overnight Culture) | |
| - | + | #Using the calculated volumes inoculate 1 x 10ml LB media in a 100ml flask that is totally covered in foil. Grow in the shaking incubator at 37<sup>o</sup>C. | |
| - | + | #After....hours of growth check the OD<sub>600</sub> of the culture using LB as a blank, if the correct OD<sub>600</sub> for exponential phase is reached then remove culture from incubator, if it has not then carry on growing for suitable length of time. | |
| - | + | #Place into a shaking incubator and grow until it reaches the exponential phase of growth, the time for this should be determined previously when we do the growth curve but always checked by removing 1ml of each culture and measuring the OD<sub>600</sub> using LB media as a blank. | |
| - | + | #Once the correct OD<sub>600</sub> has been reached then pipette 18x200μl of the ''B. subtilis'' into a 96 well plate following the plate schematic. In addition pipette 200μl of LB media into the plate. '''It is key to minimise the light exposure when loading this plate.''' | |
| - | + | #Once the plate has been loaded then carefully place sticky tape onto the top of the plate. | |
| - | + | #Place into the plate reader and open the protocol for ''characterisation of light inducible promoters'' and run protocol. | |
| - | + | #This protocol needs to be set up to use a suitable emission filter to induce the YtvA receptor. In addition we need to explore how the length of this induction and the intensity of this will affect the activation of the YtvA receptor and so we will need to find out a suitable range of these to test. | |
| - | + | #After light induction we need to set up the protocol to measure the OD<sub>600</sub> and fluorescence (for mRFP1) every 10 minutes for 6 hours. | |
| - | + | #Once data is collected dispose of the 96 well plate into the autoclaved rubbish bins (white). | |
| + | |}} | ||
| - | {{Imperial/EndPage|Protocols|}} | + | {{Imperial/EndPage|Protocols|Protocols}} |
Latest revision as of 17:44, 28 October 2008
|
|||||||
 "
"